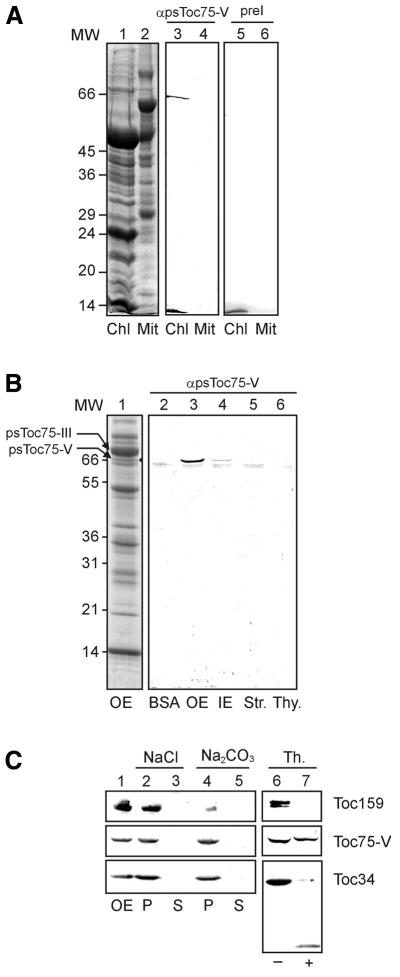
Fig. 1. Localization of a novel chloroplast protein. (A) Purified chloroplasts (Chl) or mitochondria (Mit; equivalent to 100 µg of protein) from pea leaves were separated by SDS–PAGE and either stained by Coomassie Blue (lanes 1 and 2) or immunoblotted and incubated with preimmune (lanes 3 and 4) or immune (lane 5 and 6) serum against Toc75-V. Numbers on the left indicate molecular weight (MW) markers (in kDa). (B) Different chloroplast subfractions, 15 µg of protein of each outer envelope (OE), inner envelope (IE), stroma (Str.) or thylakoids (Thy.) were used for immunoblotting. As a control, 1 µg of BSA was used to detect non-specific background. Lane 1 shows a Coomassie-stained SDS–PAGE of purified outer envelope membrane (300 µg). (C) Outer envelope membranes were either treated (+) or not treated (–) with the protease thermolysin, and the presence of Toc75-V, Toc159 and Toc34 was subsequently analysed by immunoblotting (lanes 6 and 7). Lanes 1–5 show purified outer envelopes (lane 1) extracted with either 1 M NaCl or 0.1 M Na2CO3 and separated into a soluble (S) and insoluble (P) fractions. The presence of Toc75-V, Toc159 and Toc34 was determined by immunoblotting.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.