Figure 3.
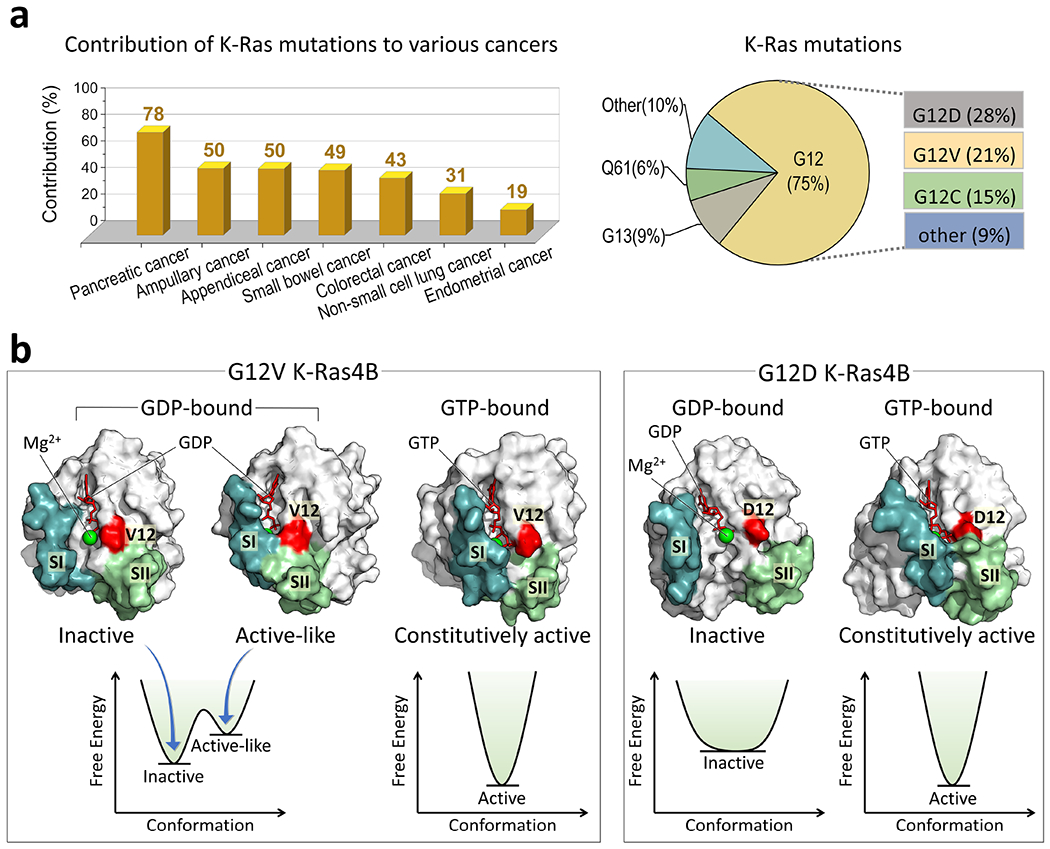
(a) K-Ras mutations can drive cancers. Analysis of the AACR cancer cohort (GENIE Cohort v13.1-public) reveals that K-Ras mutations have high associations with pancreatic, ampullary, appendiceal, small bowel, colorectal, non-small cell lung, and endometrial cancers. The most prevalent K-Ras mutation sites are at codon 12, 13, and 61, with G12 mutations being the most frequent, accounting for ~75% of all K-Ras mutations. Among these mutations, G12D is the most common (~28%), followed by G12V (~23%) and G12C (~15%). (b) K-Ras4B encompasses two critical regions, Switch I (SI) and Switch II (SII). In the inactive GDP-bound state, the two regions are separated (referred as the open SI-SII conformation), which prevents the K-Ras interactions with its effectors. In the active GTP-bound state, these two regions come into closer proximity (referred as the closed SI-SII conformation), favorable for the effector binding. Oncogenic mutations in K-Ras4B can shift the equilibrium towards the active state. The G12V and G12D mutants have a high population in the GTP-bound state with the closed SI-SII conformation, and a low population in the GDP-bound state with the open SI-SII conformation. The G12V mutation induces a more potent activation of K-Ras compared to the G12D mutation. This difference in activation strength may arise from the distinct dynamic ensembles of the two mutants. In the GDP-bound state, K-Ras carrying the most aggressive G12V mutation visits frequently the active-like conformation, featuring instances of SI and SII separation. This further amplifies the likelihood of downstream effectors binding, thereby intensifying the signal transduction.
