Figure 3. TGFB pathway is required for epithelial regeneration in the intestine after irradiation.
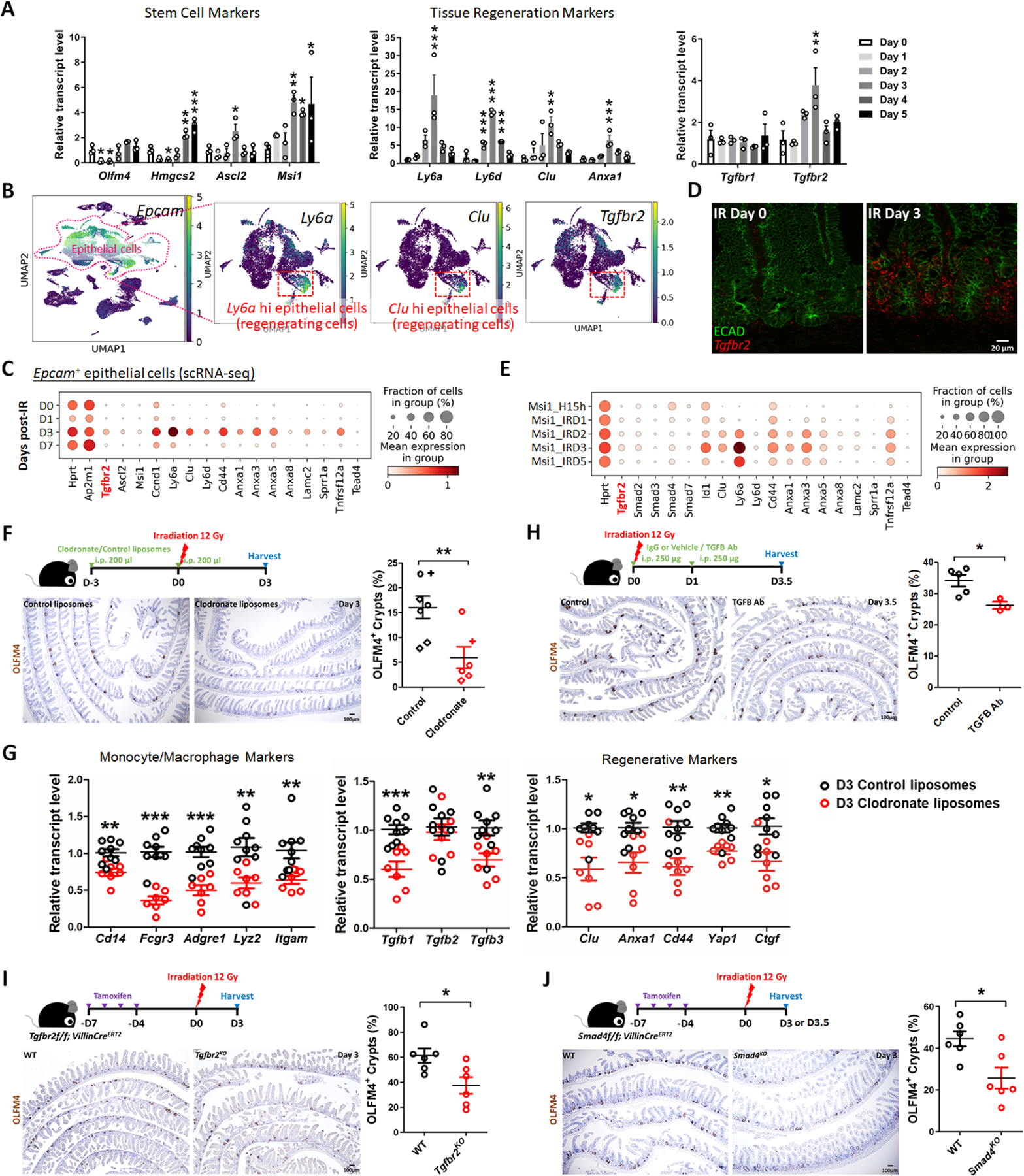
(A) qRT-PCR analysis indicates that transcript levels of stem cell marker genes, tissue regeneration marker genes and Tgfbr2 are dynamic in during intestinal regeneration post-irradiation. The qRT-PCR data are presented as mean ± SEM (n=3 biological replicates, duodenal fragments). Transcript levels relative to Day 0 before irradiation. Statistical comparisons were performed using one-way ANOVA followed by Dunnett’s post at P < 0.001***, P < 0.01** or P < 0.05*. (B) scRNA-seq of mouse intestines across a time-course post-irradiation (GSE165318). Of all the epithelial cells in the dataset (marked by Epcam expression), there is a strong correlation between Tgfbr2-expressing cells and the subset of epithelial cells expressing regenerative markers (Ly6a and Clu, see expanded panel in Figure S3A). (C) Dot plots of epithelial cells from the same dataset reveal that Tgfbr2 is highly enriched at Day 3 post-irradiation and correlated with fetal/regenerative gene profiling. Hprt and Ap2m1 were used as reference genes. (D) RNAscope reveals elevated Tgfbr2 transcripts in the Day 3 irradiated intestine (representative of 3 biological replicates). ECAD: epithelial marker. (E) An independent scRNA-seq (GSE145866 21) dataset also reveals that transcripts related to TGFB pathway and fetal/regenerative genes are elevated at Day 3 post-irradiation in sorted Msi1-GFP positive cells (irradiation-resistant) and their progeny cells. Msi1-CreERT2; R26-mTmG mice were treated with tamoxifen for 15 hours and then used as controls or further irradiation for 1, 2, 3, and 5 days. Number of cells in each condition was Msi1_H15h: n= 2281; Msi1_IRD1: n= 1257; Msi1_IRD2: n= 1312; Msi1_IRD3: n= 2989; Msi1_IRD5: n= 1792. (F) Monocytes/Macrophages were depleted using clodronate-containing liposomes (2 treatments of 200 µl i.p. injections 72 hours pre- and day of irradiation). Tissues were assessed for regenerative cell clusters using OLFM4 immunostaining. Clodronate-treated samples shows a significant reduction in the number of OLFM4 positive regenerating cell clusters. Different symbols (circle, diamond and cruciform) represent biological replicates from three different batches of experiments (n=6–7 biological replicates, distal duodenum to proximal jejunum, Student’s t-test at P < 0.01**). (G) qRT-PCR confirms downregulated transcript levels of monocyte/macrophage marker genes, Tgfb genes, and regenerative marker genes in the intestine upon clodronate treatment (n=7–9 biological replicates, duodenal fragments, Student’s t-test at P < 0.001***, P < 0.01** or P < 0.05*). Tissues were collected 3 days post-irradiation. (H) Mice treated with 2 doses of neutralizing antibodies directed against TGFB were less efficient at regenerating post irradiation compared to control-treated mice, as measured by counting the number of proliferative foci as marked by OLFM4 immunostaining (n=3–5 biological replicates, distal duodenum to proximal jejunum, Student’s t-test at P < 0.05*). IgG or vehicle treated mice were used as control mice. Tissues were collected 3.5 days post-irradiation. (I) Tgfbr2 intestine-specific knockout restricts regeneration after irradiation (n=6 biological replicates, duodenum, Student’s t-test at P < 0.05*). (J) Smad4 intestine-specific knockout restricts regeneration after irradiation (n=6 biological replicates, Jejunum, Student’s t-test at P < 0.05*). Mice were treated with tamoxifen to inactivate Tgfbr2 or Smad4 in the intestinal epithelium 7 days before 12 Gy of irradiation. Intestine was collected 3 or 3.5 days post-IR and scored for regenerative foci using OLFM4 immunostaining.
