Abstract
Pasteurella multocida is a bacterial pathogen that causes rhinitis (snuffles), pneumonia, otitis media, septicemia, metritis, and death in domestic rabbits. Currently, there are no effective vaccines to prevent infection by this organism. Subcutaneous (s.c.) immunization with either exotoxin or thiocyanate extracts of P. multocida induces partial protection in rabbits. Since disease begins at mucosal sites, induction of local immunity may be important in preventing systemic disease. Little is known concerning the efficacy of intranasal (i.n.) administration of these antigens in inducing protective mucosal immunity to P. multocida in rabbits. The purpose of this study was twofold: (i) to investigate the effectiveness of vaccination with purified P. multocida toxin (PMT) and a potassium thiocyanate extract of P. multocida (CN) in combination and (ii) to evaluate the efficacy of administration of these antigens i.n. versus s.c. Forty-eight rabbits were randomly divided into eight different treatment groups. Rabbits received either one or both antigens by either s.c. or i.n. administration. Following vaccination, each group received an i.n. challenge of P. multocida. Rabbits vaccinated with both antigens i.n. or s.c. had a 100% survival rate, few or no bacteria in the liver and lungs, high serum immunoglobulin G (IgG) and IgM antibody titers, and significant numbers of IgG antibody-secreting cells (ASC) in the spleen and tracheobronchial lymph node. Rabbits vaccinated i.n. had significant nasal and bronchoalveolar lavage IgA antibody levels. Rabbits vaccinated with only one antigen, either PMT or CN, had lower antibody titers, moderate to severe liver and lung infections, and fewer ASC compared to rabbits receiving both antigens. Rabbits in the control groups had moderate to severe liver and lung infections. This study indicates that i.n. immunization with both PMT and CN induces an effective response against homologous P. multocida challenge.
Pasteurella multocida is a common pathogen in rabbits. During stress, such as mating, shipping, and experimental handling, various serotypes of P. multocida may replicate rapidly, causing diseases such as pneumonia, otitis media, conjunctivitis, and septicemia (9, 12) and atrophic rhinitis (11). This upper-respiratory-tract pathogen is highly contagious and is readily transmitted through direct physical and aerosol contact (10), making eradication difficult. Furthermore, infections in rabbits can be caused by various toxigenic (13) and nontoxigenic serotypes of P. multocida, thus complicating vaccination strategies.
Antibiotics have been only partially successful in controlling infection, since they do not completely eliminate the bacterium (14, 18) and, like many other bacteria, P. multocida has developed resistance to some commonly used antibiotics (31). Furthermore, antibiotics are only a temporary solution to the problem because infection usually recurs within a short period of time following treatment (14).
Another potential means to control pasteurellosis is through vaccination. Attenuated live vaccines such as the Clemson University strain and the M-9 strain are currently available to prevent fowl cholera. Although these vaccines have been shown to be effective in preventing disease in turkeys and chickens (3, 8), they still have safety issues that make their use limited. For example, these attenuated vaccines have been shown to revert to their virulent wild-type state, thus causing high mortality and outbreaks of fowl cholera (16, 27) following their use. Modified live vaccines, such as the aroA mutant of P. multocida, recently showed promise in conferring heterologous immunity in mice (17). However, this vaccine has not been tested in natural target hosts, and work in this area is still under way.
Due to the safety issues plaguing the use of attenuated live vaccines, such as timing, dosage, and possible reversion to their virulent wild-type state, subunit vaccines are another alternative. Subunit vaccines can be derived from various strains of P. multocida. One such subunit is a potassium thiocyanate extract of P. multocida (CN). Subcutaneous (s.c.) administration of CN has been shown to induce considerable protection against homologous intranasal (i.n.) challenge with live organisms (19, 29). Immunization with CN is most likely effective due to the multitude of components, such as outer membrane proteins, cell wall fragments, exotoxins, and lipopolysaccharide (23), that it contains. Rabbits immunized with CN produce antibodies against outer membrane proteins and lipopolysaccharide of homologous P. multocida challenge organisms (20, 25).
Another subunit vaccine candidate is purified inactivated P. multocida toxin (PMT). Immunization of pregnant mice with PMT induces partial protection in both the mice and their offspring against homologous challenge (4, 24). i.n. immunization of rabbits with inactivated PMT stimulates PMT-specific antibodies in serum and at mucosal surfaces of the respiratory tract (28).
Vaccines containing either CN or PMT alone offer only partial protection for rabbits, as pneumonia and bacterial colonization of the nasal turbinates are still observed following challenge (20, 28, 29). Both preparations contain antigens of important virulence mechanisms; however, the efficacy of combined administration of CN and PMT has not been investigated. Combining these antigens may produce superior protective immunity. Since P. multocida infections colonize the upper respiratory tract, the mucosal immune response is likely to be an important defense mechanism. Secretory IgA (sIgA) antibodies are abundant in mucosal secretions and function to inhibit microbial adherence to epithelial cells (22). sIgA is preferentially induced following mucosal immunization; thus, the production of sIgA following i.n. vaccination should help prevent bacterial colonization and subsequent infection.
The objective of this research was twofold: (i) to determine if coadministration of CN and PMT offers better protection against pasteurellosis in New Zealand White male rabbits than either one given alone and (ii) to evaluate the efficacy of i.n. versus s.c. administration in stimulating protective immunity.
MATERIALS AND METHODS
Experimental animals.
Forty-eight New Zealand White male rabbits (Oryctolagus cuniculus) (Hazleton Research Products, Inc., Kalamazoo, Mich.) weighting 2 to 2.5 kg were used. The colony from which the rabbits were obtained was Pasteurella free. Rabbits were placed in individual stainless steel cages upon arrival and allowed to acclimate to their environment for 5 days. Commercial feed (Purina Lab Rabbit Chow 5321; PMI Inc., Richmond, Ind.) and tap water were supplied ad libitum. The use of rabbits in this study was authorized by the Purdue University Animal Care and Use Committee.
CN.
Extracts were prepared from P. multocida 3,12,15:D, isolated from the bone marrow of an infected rabbit (29). This isolate produced heat-labile toxin, as confirmed by a tissue culture assay with bovine fetal lung cells and CN (Oxford Laboratories, Worthington, Minn.) and by use of a DNA molecular probe for the dermonecrotoxin gene (assay performed by S. Singha, Breathitt Veterinary Center, Hopkinsville, Ky.). CN was prepared as previously described (25). Briefly, P. multocida was grown to confluence on 5% horse blood agar (Becton Dickinson, Cockeysville, Md.) in a 37°C CO2 incubator for 24 h. After 24 h of incubation, 6 ml of equal parts phosphate-buffered saline (PBS, pH 7.2) and 1 M potassium thiocyanate (KSCN) (Fisher Scientific Co., Pittsburgh, Pa.) was added to each bacterial plate.
A cotton-tip swab was used to scrape the bacteria off the plate, and the suspension was placed into a flask. The flask was placed in a 37°C shaking water bath for 6 h. The bacterial suspension was centrifuged at 8,000 × g for 10 min at 10°C to remove cell debris. The supernatant was collected and dialyzed over a 3-day period at 4°C with 0.01 M Tris hydrochloride (pH 8), which was changed every 6 to 12 h. The extract was concentrated by ultrafiltration with Centriprep-10 concentrators (Millipore Corp., Bedford, Mass.) with a nominal molecular weight limit of 10,000. CN was sterilized by filtration through a 0.2-μm-pore-size filter (Amicon, Inc., Beverly, Mass.). The protein concentration of CN was determined by use of a bicinchoninic acid assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin (BSA) as the protein standard. In general, seven bacterial plates grown to confluence will yield 1 ml of CN at a concentration of 1 mg/ml, and in this study 70 plates were used.
Inactivation and preparation of CN for inoculation.
CN (1 mg/ml) was toxic at dilutions as low as 1:1,000 when assayed with embryonic bovine lung cells. Therefore, CN was inactivated in a 60°C water bath for 30 min before it was given to rabbits (29). Each dose of CN, whether administered i.n. or s.c., was prepared for each rabbit on the day of inoculation and was given in 1-ml aliquots. For i.n. administration of vaccines containing CN, 1-ml doses contained 20 μg of cholera toxin (CT) (1 mg/ml) (Sigma Chemical Co., St. Louis, Mo.), added as a mucosal adjuvant (21, 29), and 1 mg of inactivated CN dissolved in PBS (pH 7.2). For s.c. administration of vaccines containing inactivated CN, aluminum hydroxide [Al(OH)3] was used as the adjuvant. CN (1 mg/ml) was mixed with 500 μl of 1 M NaHCO3 and 1 ml of 0.2 M AlK(SO4)2 and vortexed. The mixture was microcentrifuged for 5 min (13,000 × g) to remove the supernatant. The pellet was washed three times with PBS, and the final pellet was brought to 1 ml with PBS.
Inactivation and preparation of PMT for inoculation.
On the day of inoculation, each 1-ml dose of PMT, whether administered i.n. or s.c., was prepared separately for each rabbit. One hundred microliters (5 μg/ml) of purified PMT (generous gift of Richard Rimler, Ames, Iowa) was placed in a 1.5-ml microcentrifuge tube and inactivated in a 60°C water bath for 30 min. For i.n. administration of vaccines containing PMT, 1-ml doses contained 5 μg of inactivated PMT and 20 μg of CT (1 mg/ml) in sterile saline. For s.c. administration of vaccines containing PMT, 1 ml of PMT (50 μg) was inactivated, mixed with 22.5 μl of 1 M NaHCO3 and 50 μl of 0.2 M AlK(SO4)2, and vortexed. The suspension was microcentrifuged at 13,000 × g for 5 min. The supernatant was discarded, and the remaining pellet was washed three times with PBS and resuspended by vortexing in 1 ml of PBS.
Treatment groups.
Rabbits were randomly assigned to one of eight different groups. Two groups served as controls, receiving only saline or Al(OH)3, and the other six received inoculations containing either PMT or CN alone or in combination with each other (PMTCN) via the i.n. or s.c. route. Groups were as follows: inPMT, i.n. PMT plus CT; scPMT, s.c. PMT plus Al(OH)3; inCN, i.n. CN plus CT; scCN, s.c. CN plus Al(OH)3; inPMTCN, i.n. PMT plus CN plus CT; scPMTCN, s.c. PMT plus CN plus Al(OH)3; inCo, i.n. control (saline); and scCo, s.c. control [Al(OH)3]. Groups receiving any form of CN were vaccinated on days 7, 21, and 35. Groups receiving any form of PMT were vaccinated on days 7 and 35. Groups receiving PMT plus CN were given CN on days 7, 21, and 35 and PMT on days 7 and 35.
Each trial included eight rabbits, each representing one of the eight different treatment groups and each housed in a separate cage, four rabbits per room. The trial lasted for roughly 8 weeks, and only four rabbits were challenged at one time due to the labor-intensive task of collecting and analyzing samples. At no time were the infected rabbits exposed to the uninfected rabbits. This 8-week study was repeated six separate times. All rabbits were challenged i.n. on day 49 with about 106 CFU of viable P. multocida in 1 ml of saline, split equally between both nares, and were necropsied on day 53.
Sample collection.
Blood was collected from the lateral ear vein. Nasal washings were collected by tilting a rabbit’s head to one side, injecting 1 ml of saline gently into the upturned naris with a feeding needle, and allowing the washings to drip from the contralateral naris into a petri dish. This process was repeated for the other side. Washings were pooled and centrifuged at 200 × g for 10 min to remove any cell debris, and the supernatant was placed in a tube and frozen at −20°C until analyzed. Blood and nasal wash samples were taken (i) prior to inoculation (day 0), (ii) 1 week following each inoculation (days 14, 28, and 42), and (iii) 3 days following challenge with virulent P. multocida (day 52). Essentially, blood and nasal wash samples were collected on days 0, 14, 28, 42, and 52.
Preparation and confirmation of challenge dose.
Bacteria were diluted to an absorbance value equal to 109 CFU/ml on a standard curve and then diluted to the desired dose on the day of challenge. Counts were confirmed by serially diluting the challenge preparations and culturing them on blood agar plates. A challenge dose of 106 CFU/ml was determined to be the target dose based on previous studies (28, 29). Challenge doses were 106 CFU/ml for four trials and 105 and 107 CFU/ml for one trial each. Although the challenge doses varied somewhat, this fact neither added a significant amount of experimental variation nor resulted in significant differences between the six separate challenge trials.
Postmortem examination.
Rabbits were sedated with ketamine-xylazine (Fort Dodge Laboratories Inc., Fort Dodge, Iowa) and euthanatized by an intravenous overdose of sodium pentobarbital (Butler Co., Columbus, Ohio). The spleen, mesenteric lymph node (MLN), tracheobronchial lymph node (TLN), and both tonsils were collected for ELISpot assays. Intact lungs were removed and lavaged via the bronchi with 5 ml of sterile PBS. Lavage samples were centrifuged to remove cellular debris and stored at −20°C for later analysis. P. multocida from the liver, lungs, right and left bullae, and nasal pharynx was enumerated by culturing, and sections of liver and lungs were taken for histologic examination.
Liver and lung cultures.
Liver and lung samples were collected and weighed, an equivalent amount (weight/volume) of sterile saline was added, and each organ was macerated with a stomacher (Seward Medical, London, England). One milliliter of the tissue supernatant was added to 9 ml of sterile saline and vortexed. Serial dilutions of the tissue samples were plated on blood agar plates and grown overnight at 37°C. The numbers of CFUs of P. multocida per gram of tissue were recorded. The identity of organisms recovered from cultures was confirmed with the API 20E system (Biomerieux, St. Louis, Mo.).
Pathologic and histologic evaluations.
At necropsy, each lung lobe was scored for percentage of pneumonic lung. The lungs were palpated, and the area of affected lobes (right and left cranial, middle, and caudal) was recorded. Histologic lesion scores were evaluated as previously described (5). After collection of a section of the lung for bacterial culturing, the lung was fixed in 10% neutral buffered formalin. One section each of the right and left lungs was cut from the cranial and caudal lobes and embedded in paraffin. Six-micrometer hematoxylin-and-eosin-stained sections were evaluated for hemorrhage, necrosis, fibrin formation, pleuritis, bacterial colonies, and cellular infiltrates in the bronchioles, interstitium, and alveolar spaces. For the presence of each criterion, a value of 1 was given; otherwise, a value of 0 was assigned. The results for each right and left lung section were combined to give a lesion score ranging from 0 (not severe) to 36 (most severe). The percentage of grossly abnormal lung was multiplied by the histologic lesion score to generate a pathology score.
Preparation of cells for ELISpot assay.
At necropsy, the spleen, MLN, TLN, and tonsils were placed in Iscove’s medium containing 10% fetal clone I (Hyclone Laboratories, Inc., Logan, Utah) and 1% penicillin-streptomycin-amphicin (Sigma) and then macerated with sterile forceps. The medium was spun at 200 × g for 10 min, and the cell pellet was washed twice with cell medium and resuspended in complete medium. A sample of the cell suspension was stained with trypan blue exclusion dye, and the number of live cells was estimated with a hemocytometer.
ELISpot assay.
Enzyme immunoassay-radioimmunoassay flat-bottom high-binding 96-well plates (Costar Corp., Cambridge, Mass.) were coated with 2.5 μg of inactivated CN per ml in PBS (100 μl/well) and incubated overnight at 4°C. On the following day, plates were washed three times with PBS–0.05% (vol/vol) Tween 20 (PBS-Tween) and blocked with 100 μl of PBS–0.01% BSA per well at 37°C for 1 h. Plates were washed twice with PBS-Tween and once with PBS, and 100 μl of 105 cells from each tissue was added per well. Plates were placed at 37°C for 4 h and washed three times with PBS-Tween. Goat anti-rabbit IgA, IgG, and IgM sera (Southern Biotechnology Associates, Birmingham, Ala.) were diluted to an optimal working concentration (1:20,000) with PBS-Tween and absorbed with a 1:1,000 dilution of inactivated CN. They were then incubated overnight at 4°C.
On the following day, plates were washed three times with PBS-Tween, and rabbit anti-goat IgG (heavy and light chains) conjugated to alkaline phosphatase (Southern Biotechnology) was diluted to 1:1,000 with PBS-Tween and absorbed with inactivated CN. Seventy-five microliters of conjugated antibody was added to each well. Plates were incubated for 3 h at room temperature and washed three times with PBS-Tween. Substrate was prepared by diluting 1 part 3% (wt/vol) agarose (ultrapure, high melting temperature; Gibco BRL, Grand Island, N.Y.) with 4 parts 5-bromo-4-chloro-3-indolylphosphate (5-BCIP) (Sigma). The agarose was first melted in a boiling water bath and then mixed with 5-BCIP. The mixture was kept at 40°C by placing the tube containing the mixture in a beaker containing hot water. One hundred microliters of the warm mixture was added to each well. Plates were incubated for 10 min at room temperature and placed in a humid chamber overnight at 4°C. Spots were counted with a PhotoZoom inverted microscope (Cambridge Instruments), and the number of spots per six wells was averaged and recorded as the mean number of antibody-secreting cells (ASC) per 106 cells.
ELISA.
Enzyme immunoassay-radioimmunoassay flat-bottom high-binding 96-well plates were coated with 100 μl of inactivated CN (2.5 μg/ml) per well as described above for the ELISpot assay. This quantity of antigen was chosen because it gave optimal enzyme-linked immunosorbent assay (ELISA) readings for rabbit serum samples. On the following day, plates were washed and blocked with 0.01% BSA as described above. Plates were washed three times with PBS-Tween. serum samples were serially diluted from 1:100 to 1:6,400 and assayed in duplicate.
Each ELISA plate included six negative control wells and six positive control wells. Negative controls consisted of pooled sera obtained from six unimmunized rabbits and used at a dilution of 1:100 with PBS-Tween (50 μl/well). For positive controls, sera obtained from six rabbits with high anti-CN titers were pooled and diluted to 1:100 with PBS-Tween.
Plates were incubated for 2 h at 37°C and washed three times with PBS-Tween. Goat anti-rabbit IgA, IgG, and IgM sera were diluted 1:20,000 with PBS-Tween and absorbed with inactivated CN at a 1:1,000 dilution. Fifty microliters of each isotype was added to each well of the plate and incubated overnight at 4°C. Plates were washed three times with PBS-Tween, and 50 μl of rabbit anti-goat IgG (heavy and light chains) conjugated to alkaline phosphatase, diluted 1:1,000 in PBS-Tween, and absorbed with inactivated CN was added to each well. Plates were incubated for 3 h at room temperature and washed three times with PBS-Tween, and 50 μl of the substrate p-nitrophenyl phosphate (Sigma) (1 mg/ml) was added to each well and incubated for 15 min at 25°C.
The absorbance of each well was recorded at 405 nm with a 96-well microplate reader (Molecular Devices, Menlo Park, Calif.). Results for serum were reported as end-point antibody titers. The titer was designated the reciprocal of the last dilution of serum more than two standard deviations from the mean of the negative control samples. Samples which were below the detection level (i.e., were less than 1:100, or a value of 100) were considered negative and were assigned a value of 50 (i.e., 1:50) (30). To normalize the data and permit valid statistical evaluation, each value was transformed to the geometric log2 value.
Nasal lavage samples were diluted 1:6 and bronchoalveolar lavage fluids (BAL) were diluted 1:2 with PBS-Tween and assayed by an ELISA without end-point dilutions. Each BAL and nasal wash ELISA plate also included six negative control wells and six positive control wells. The negative controls consisted of PBS-Tween, and the positive controls consisted of serum-positive samples diluted 1:100 in PBS-Tween. Samples were considered positive if their average optical density (OD) was 2 standard deviations from the negative control mean. Results for nasal wash and BAL samples were reported as percentages of positive samples, calculated with the following formula: [(average sample OD − average negative control OD)/(average positive control OD − average negative control OD)] × 100. Since the positive control OD reflects an average for several positive rabbits, it is possible for a positive sample to have a higher OD than the average and thus exceed 100%.
Statistical analysis.
All results were analyzed with Statistica for Windows (StatSoft, Tulsa, Okla.). Multivariate analysis of variance (MANOVA) was used to analyze between- and within-group interactions for serum, nasal wash, BAL, and ELISpot results, with a P value of <0.05 being considered significant. Within-group interaction took into consideration the repetitive measure (antibodies) for each treatment group at each sampling period. Analysis of variance (ANOVA) was used to analyze between-group interaction for severity of pneumonia and CFU of P. multocida recovered from liver and lungs, with a P value of <0.05 being considered significant.
RESULTS
Survival rate after challenge with P. multocida.
Following challenge, 100% of rabbits in the inPMT, scPMT, inCN, inPMTCN, and scPMTCN groups survived. However, only 50% of rabbits in the scCN group, 83% of rabbits in the inCo group, and 0% of rabbits in the scCo group survived to day 53. All rabbits in the scCO group had severe pneumonia and purulent discharge from the nostrils and eyes, while rabbits in the inCo group displayed signs of lethargy, anorexia, and discharge from the nostrils. With the exception of rabbits in the scCN group, rabbits immunized with either PMT or CN survived challenge and did not appear to develop nasal or ocular discharge.
P. multocida cultured from the liver and lungs of challenged rabbits.
The numbers of CFUs of P. multocida cultured from the liver and lungs of each rabbit were converted to log10 values (Tables 1 and 2). Rabbits in the inPMTCN group and the scPMTCN group had no P. multocida cultured from the liver. Furthermore, the two PMT- and CN-vaccinated groups had significantly fewer CFU recovered from the liver than the scCo group (P < 0.03).
TABLE 1.
Mean log10 CFU of P. multocida per g of tissue recovered from the liver of challenged rabbitsa
| Treatment group | Mean log10 CFU/g of liver | Statistical significance (P) of difference between groups
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| inPMT | scPMT | inCN | scCN | inPMTCN | scPMTCN | inCo | scCo | ||
| inPMT | 2.3 | 0.04 | 0.04 | 0.01 | |||||
| scPMT | 0.2 | 0.02 | 0.01 | ||||||
| inCN | 1.4 | 0.01 | |||||||
| scCN | 3 | 0.01 | 0.01 | 0.03 | |||||
| inPMTCN | 0 | 0.04 | 0.01 | 0.01 | |||||
| scPMTCN | 0 | 0.04 | 0.01 | 0.01 | |||||
| inCo | 2 | ||||||||
| scCo | 5.5 | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 | ||
See the legend to Fig. 1 for details. ANOVA was used to analyze between-group interactions, with a P value of <0.05 being considered significant. Individual P values are shown.
TABLE 2.
Mean log10 CFU of P. multocida per g of tissue recovered from the lungs of challenged rabbitsa
| Treatment group | Mean log10 CFU/g of lung | Statistical significance (P) of difference between groups
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| inPMT | scPMT | inCN | scCN | inPMTCN | scPMTCN | inCo | scCo | ||
| inPMT | 2.8 | 0.01 | 0.01 | ||||||
| scPMT | 2.9 | 0.01 | 0.01 | ||||||
| inCN | 3.4 | 0.01 | 0.03 | 0.04 | |||||
| scCN | 3.9 | 0.01 | 0.01 | ||||||
| inPMTCN | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| scPMTCN | 0.7 | 0.03 | 0.01 | 0.02 | 0.01 | ||||
| inCo | 3.4 | 0.01 | 0.02 | ||||||
| scCo | 5.9 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | |||
See the legend to Fig. 1 for details. ANOVA was used to analyze between-group interactions, with a P value of <0.05 being considered significant. Individual P values are shown.
In contrast to rabbits in the inPMTCN and scPMTCN groups, which had the lowest numbers of CFUs of P. multocida recovered from the lungs (Table 2), rabbits in the scCN, inCN, inCo, and scCo groups had the largest amounts of P. multocida cultured from the lungs (P < 0.02).
P. multocida-positive cultures from the bullae and nasal pharynx following challenge.
P. multocida was cultured from the bullae of none of the rabbits in the scPMTCN group, 16% in the inPMTCN group, 40% in the inCo group, 50% in the inCN, scCN, and inPMT groups, and 60% in the scPMT group. When the nasal pharynx was cultured, the inPMTCN group had the lowest culture-positive rate (33%), followed by the inCo group (80%), the inPMT and scPMTCN groups (83%), and the scPMT, inCN, and scCN groups (100%). No data were collected for the scCo group, since all of the rabbits died 3 days postchallenge. In general, rabbits which were vaccinated with both antigens had the lowest culture-positive rates.
Summary of lung lesions.
Lung lesion scores were ranged according to severity. The inPMTCN group had the lowest severity score (0.1), followed by the scPMTCN group (0.2), the scPMT group (0.5), the inCN group (2.8), the inPMT group (3.6), the inCo group (4.4), the scCN group (10.3), and the scCo group (17.5). Rabbits in the scCN and scCo groups had significantly higher lesion severity scores (P < 0.02) than those in all the other groups.
Serum end-point antibody titers.
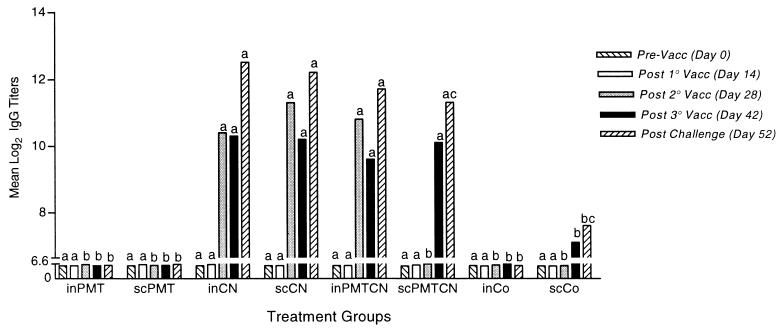
Following the second vaccination period, rabbits receiving CN, either alone or in combination with PMT, had an increased IgG level (Fig. 1), and most rabbits receiving CN had an increase followed by a decrease in the IgM level (data not shown). No group had a significant increase in serum IgA antibody titers over time (data not shown). Control rabbits or rabbits receiving PMT alone had little or no increase in anti-CN antibody titers following each subsequent immunization. Following challenge with live P. multocida, rabbits receiving any form of CN had an increase in the IgG level (Fig. 1). Furthermore, rabbits which received any form of CN had an average twofold-higher IgG level than control rabbits or rabbits receiving PMT alone (P < 0.05).
FIG. 1.
Mean log2 serum anti-CN IgG antibody titers. Rabbits were vaccinated (on days 7, 21, and 35, except for groups receiving PMT, which were vaccinated on days 7 and 35) either i.n. or s.c. with either PMT or CN alone or in combination with each other (PMTCN) or with a control (Co) (aluminum hydroxide for s.c. administrations and saline for i.n. administrations). Serum samples were collected prior to vaccination, 1 week after each vaccination, and 3 days following challenge. Rabbits were challenged i.n. with virulent P. multocida on day 49. Samples were analyzed by an ELISA as described in Materials and Methods. Between- and within-group interactions were analyzed by MANOVA, with a P value of <0.05 being considered statistically significant. Lowercase letters signify comparisons made between groups, with different letters signifying statistical significance between groups at each time period. Vacc, vaccination.
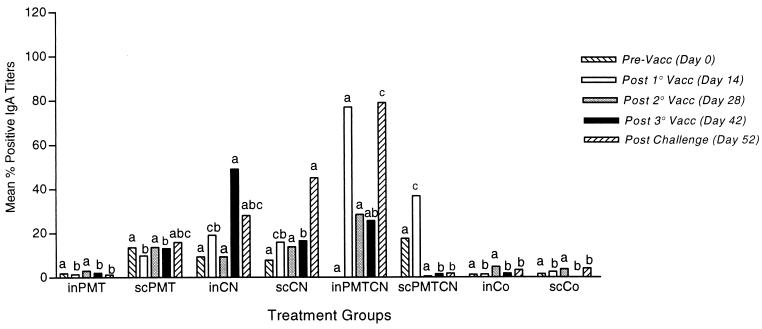
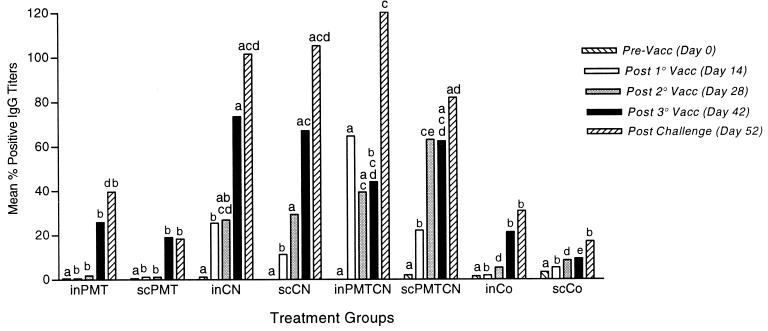
Percent positive nasal wash antibody titers.
Preimmunization titers were not significantly different between groups for any isotype. The inPMTCN group had the greatest IgA response of all groups both after the first inoculation and following challenge (Fig. 2). The greatest increase in IgG antibody levels was for rabbits vaccinated i.n. or s.c. with CN or PMTCN (Fig. 3), while only rabbits receiving both antigens had a significant increase in IgM antibody levels (P < 0.04) (data not shown).
FIG. 2.
Mean percent positive nasal anti-CN IgA antibody titers. Rabbits were vaccinated and samples were collected as described in the legend to Fig. 1. Samples were analyzed by an ELISA, and between- and within-group interactions were determined by MANOVA, with a P value of <0.05 being considered statistically significant. Vacc, vaccination.
FIG. 3.
Mean percent positive nasal anti-CN IgG antibody titers. Rabbits were vaccinated and samples were collected as described in the legend to Fig. 1. Samples were analyzed with an ELISA, and between- and within-group interactions were determined by MANOVA, with a P value of <0.05 being considered statistically significant. Vacc, vaccination.
Throughout the study, the inPMTCN group consistently had higher IgA and IgG antibody titers than all the other groups, except for the third immunization, after which the inCN group had the highest IgA and IgG antibody titers (Fig. 2 and 3). Following challenge with P. multocida, the inPMTCN group had the highest IgA and IgG antibody titers. In general, rabbits which received any form of CN had higher IgA and IgG antibody titers than rabbits in the two PMT and control groups (P < 0.05).
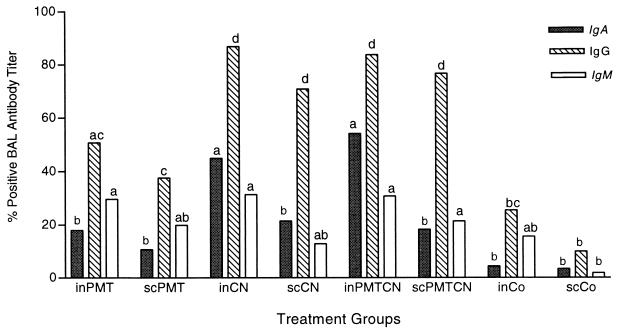
BAL isotype-specific antibody titers.
Rabbits receiving CN or PMTCN i.n. or s.c. had the highest levels of IgG antibodies in BAL (P < 0.04) (Fig. 4). The inCN and inPMTCN groups had a significant (P < 0.05) increase in IgA antibody levels, approximately three times higher than in the other groups. The inPMT, inCN, inPMTCN, and scPMTCN groups had the highest levels of IgM antibodies.
FIG. 4.
Mean percent positive BAL anti-CN IgA, IgG, and IgM antibody titers. See the legend to Fig. 1 for a description of the study. Four days following challenge with live P. multocida, BAL was collected for each rabbit. ELISA was used to analyze lavage samples, and MANOVA was used to determine between- and within-group interactions, with a P value of <0.05 being considered statistically significant.
ELISpot assay results.
The numbers of spots detected in the ELISpot assay were averaged and recorded as the number of ASC per 106 tissue cells. No significant amounts of ASC were produced in the MLN or tonsils of any group for any isotype. However, differences were detected for the spleen and TLN. Rabbits receiving both antigens had significantly more anti-CN IgG ASC in the spleen than rabbits in all the other groups (Table 3). The number of anti-CN IgG ASC produced in the TLN by the scCN group was larger than that in any other group (Table 4). Furthermore, the inPMT, inCN, inPMTCN, and scPMTCN groups had a significantly larger number of anti-CN IgG ASC in the TLN than the scPMT group. The inPMTCN group was the only group to produce a significant amount of anti-CN IgM ASC in the spleen (127 ASC/106 spleen cells; P < 0.01), 3 to 12 times more than any other group (data not shown).
TABLE 3.
Mean number of anti-CN IgG ASC per 106 spleen cells produced in response to challenge with virulent P. multocidaa
| Treatment group | Mean no. of IgG ASC/106 spleen cells | Statistical significance (P) of difference between groups
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| inPMT | scPMT | inCN | scCN | inPMTCN | scPMTCN | inCo | scCo | ||
| inPMT | 59 | 0.01 | 0.01 | 0.01 | |||||
| scPMT | 19 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| inCN | 79 | 0.01 | 0.02 | 0.01 | 0.01 | ||||
| scCN | 155 | 0.01 | 0.01 | 0.02 | 0.02 | ||||
| inPMTCN | 196 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| scPMTCN | 164 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| inCo | 75 | ||||||||
| scCo | NA | 0.02 | 0.01 | 0.01 | |||||
See the legend to Fig. 1 for details. MANOVA was used to analyze between- and within-group interactions, with a P value of <0.05 being considered significant. Individual P values for between-group comparisons are shown. NA, rabbits did not survive to day of necropsy, and no data were available.
TABLE 4.
Mean number of anti-CN IgG ASC per 106 TLN cells produced in response to challenge with virulent P. multocidaa
| Treatment group | Mean no. of IgG ASC/106 TLN cells | Statistical significance (P) of difference between groups
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| inPMT | scPMT | inCN | scCN | inPMTCN | scPMTCN | inCo | scCo | ||
| inPMT | 105 | 0.01 | 0.05 | 0.01 | 0.03 | ||||
| scPMT | 26 | 0.01 | 0.02 | 0.01 | |||||
| inCN | 89 | 0.02 | 0.02 | ||||||
| scCN | 178 | 0.05 | 0.01 | 0.02 | 0.02 | 0.01 | |||
| inPMTCN | 107 | 0.01 | |||||||
| scPMTCN | 87 | 0.03 | |||||||
| inCo | 72 | ||||||||
| scCo | NA | ||||||||
See the legend to Fig. 1 for details. MANOVA was used to analyze between- and within-group interactions, with a P value of <0.05 being considered significant. Individual P values for between-group comparisons are shown. NA, rabbits did not survive to day of necropsy, and no data were available.
DISCUSSION
One of the most difficult aspects of pasteurellosis and other P. multocida-related infections is that they can be caused by various toxigenic and nontoxigenic serotypes of the bacterium. Most infections are caused by type A P. multocida, but type D is also a prominent serotype responsible for the development of atrophic rhinitis in pigs and rabbits (13). Due to the ability of various serotypes to induce disease, vaccination becomes difficult and immunity against one serotype does not necessarily result in cross-protection against a heterologous serotype. However, this is the first study to demonstrate that coadministration of PMT and CN antigens stimulates homologous protective immunity to pasteurellosis in rabbits. Rabbits which were vaccinated with both antigens, whether by i.n. or s.c. inoculation, had the best protection, as indicated by high survival rates, few or no P. multocida organisms cultured from the middle ear, liver, or lungs, and minimal histologic lung lesions.
Protection cannot be directly linked to any one isotype or antibody response, since several groups had similar serum, nasal wash, or BAL titers. This finding suggests a possible role for cell-mediated immunity. Enhanced alveolar macrophage or T-cell responses are important for the clearance of bacterial infections (7, 26), and this study supports previous findings which demonstrated a direct correlation between decreased bacterial load and enhanced survival of challenged animals (7).
Rabbits vaccinated with PMT alone, whether s.c. or i.n., still showed partial protection against challenge, as indicated by low lung lesion scores and high survival rates, supporting the results of previous studies (28). However, these groups had more bacteria cultured from their tissues than those which received both antigens, indicating that PMT and CN together induce greater protective immunity.
In general, rabbits which were immunized, regardless of antigen or route of administration, had a 100% survival rate following challenge with homologous P. multocida, with the exception of the scCN group, which had only a 50% survival rate. It is surprising to find that the scCN group had such a low survival rate and severe lung lesions, given that the rabbits in this group had high antibody titers. However, samples were analyzed for the whole isotype present and not for the specific subclass of an isotype produced in response to the CN antigen. Therefore, even though the rabbits in the scCN group had high serum IgG antibody titers, they may not have mounted the appropriate subclass of IgG to opsonize P. multocida efficiently. Poor opsonization could lead to severe systemic infections by the bacterium. Lack of effective cell-mediated immunity could also be a factor. Previous studies have shown that the administration of antigen stimulates macrophage activity and results in greater protection against challenge by P. aeruginosa in rats (7).
Rabbits in the scCo group had the largest amount of P. multocida cultured from the liver and lungs. They also had one of the most severe lung lesion scores and a 0% survival rate, while the inCo group had an 83% survival rate. The high survival rate of the inCo group suggests that these rabbits were partially protected. The reason for this result is not obvious, but it is possible that i.n. inoculation with a feeding needle results in minor tissue damage and the release of cytokines that provide partial, nonspecific protection. Another possibility is that a few rabbits in the inCo group were exposed to PMT via a gastric feeding needle which was used to immunize the inPMT rabbits. However, none of the control animals had significant levels of CN-specific antibodies in serum, nasal wash, or BAL samples. Even though survivors in the inCo group had some anti-CN IgG ASC in the spleen and TLN, they were ill at the time of necropsy, displaying signs of lethargy, anorexia, and nasal discharge. Nonspecific immune features were most likely responsible for the decrease in bacterial numbers and the increase in the survival rate of the inCo group.
High levels of IgA antibodies were present in nasal secretions of the inPMTCN group and in BAL secretions of the inCN and inPMTCN groups. This finding confirms those of numerous studies showing that mucosal but not s.c. inoculation results in the production of IgA antibodies (6, 29). i.n. immunization causes an increase in IgA antibody levels in the nasally associated lymphoid tissue (NALT) in the upper respiratory tract (32) as well as in the bronchially associated lymphoid tissue in the lower respiratory tract (1, 2, 15). These two inductive sites allow for isotype switching and differentiation of B cells to IgA-secreting cells. NALT plays an important role in that it has the capacity to aid in B-cell maturation and differentiation as well as to maintain immune memory (32). Furthermore, the presence of IgA antibodies has been shown to be important for blocking bacterial attachment and antigen uptake across mucosal membranes (22). If the bacteria can be sequestered and destroyed at the site of infection, then further systemic damage and possible death can be prevented. However, the presence of IgA alone does not correlate highly with protection, as both i.n. and s.c. immunizations induced protective immunity in rabbits.
Even though i.n. immunization with PMTCN is an effective way to control infection, the method of vaccine delivery is not necessarily practical, especially when vaccinating a large number of rabbits. However, the efficacy of mucosal vaccination suggests that it may eventually be possible to deliver these antigens by alternative routes, such as orally, to induce mucosal immunity in respiratory tracts of rabbits (29). Furthermore, oral delivery of a vaccine could provide a safe, practical, and effective means of protecting rabbits without concern for the development of injection site reactions or discomfort from i.n. administration.
ACKNOWLEDGMENTS
This work was supported by the American Rabbit Breeders Association.
We thank Max J. Freeman for editorial efforts in preparing the manuscript.
REFERENCES
- 1.Bienenstock J, Johnston N, Perey D Y E. Bronchial lymphoid tissue. II. Functional characteristics. Lab Invest. 1973;28:693–698. [PubMed] [Google Scholar]
- 2.Bienenstock J. Bronchus-associated lymphoid tissue. In: Bienenstock J, editor. Immunology of the lung and upper respiratory tract. New York, N.Y: McGraw-Hill Book Co.; 1984. pp. 96–118. [Google Scholar]
- 3.Bierer B W, Derieux W T. Immunologic response of turkeys to an avirulent Pasteurella multocida vaccine in the drinking water. Poult Sci. 1972;51:408–416. doi: 10.3382/ps.0510408. [DOI] [PubMed] [Google Scholar]
- 4.Bording A, Foged N T. Characterization of the immunogenicity of formaldehyde detoxified Pasteurella multocida toxin. Vet Microbiol. 1991;29:267–280. doi: 10.1016/0378-1135(91)90134-2. [DOI] [PubMed] [Google Scholar]
- 5.Bowersock T L, Shalaby W S, Levy M, Samuels M L, Lallone R, White M R, Borie D L, Lehmeyer J, Park K. Evaluation of an orally administered vaccine, using hydrogels containing bacterial exotoxins of Pasteurella hemolytica, in cattle. Am J Vet Res. 1994;55:502–509. [PubMed] [Google Scholar]
- 6.Butler J E, Swanson P A, Richerson H B, Ratajczak H V, Richards D W, Suelzer M T. The local and systemic IgA and IgG antibody responses of rabbits to a soluble inhaled antigen. Am Rev Respir Dis. 1982;126:80–85. doi: 10.1164/arrd.1982.126.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Cripps A W, Dunkley M L, Clancy R L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun. 1994;62:1427–1436. doi: 10.1128/iai.62.4.1427-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis R B. Cholera and broiler breeders. Poult Sci. 1987;20:430–434. [Google Scholar]
- 9.DiGiacomo R F, Garlinghouse L E, Van Hoosier G L. Natural history of infection with Pasteurella multocida in rabbits. J Am Vet Med Assoc. 1983;183:1172–1175. [PubMed] [Google Scholar]
- 10.DiGiacomo R F, Jones C D R, Wathes C M. Transmission of Pasteurella multocida in rabbits. Lab Anim Sci. 1987;37:621–623. [PubMed] [Google Scholar]
- 11.DiGiacomo R F, Deeb B J, Giddens W E, Jr, Bernard B L, Chengappa M M. Atrophic rhinitis in New Zealand White rabbits infected with Pasteurella multocida. Am J Vet Res. 1989;50:1460–1465. [PubMed] [Google Scholar]
- 12.Flatt R E. Bacterial disease: pasteurellosis. In: Weisbroth H, Flatt R E, Kraus A L, editors. The biology of the laboratory rabbit. New York, N.Y: Academic Press, Inc.; 1974. pp. 194–205. [Google Scholar]
- 13.Foged N T. Pasteurella multocida toxin: the characterisation of the toxin and its significance in the diagnosis and prevention of progressive atrophic rhinitis in pigs. APMIS Suppl. 1992;25:1–56. [PubMed] [Google Scholar]
- 14.Gaertner D J. Comparison of penicillin and gentamicin for treatment of pasteurellosis in rabbits. Lab Anim Sci. 1991;41:78–80. [PubMed] [Google Scholar]
- 15.Gehrke I, Pabst R. The epithelium overlying rabbit bronchus-associated lymphoid tissue does not express the secretory component of immunoglobulin A. Cell Tissue Res. 1990;259:397–399. doi: 10.1007/BF00318464. [DOI] [PubMed] [Google Scholar]
- 16.Hofacre C L, Glisson J R. A serotypic survey of Pasteurella multocida isolated from poultry. Avian Dis. 1986;30:632–633. [PubMed] [Google Scholar]
- 17.Homchampa P, Strugnell R A, Adler B. Molecular analysis of the aroA gene of Pasteurella multocida and vaccine potential of a constructed aroA mutant. Mol Microbiol. 1997;6:3585–3593. doi: 10.1111/j.1365-2958.1992.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 18.Jaslow B W, Ringler D H, Rush H G, Glorioso J C. Pasteurella associated rhinitis of rabbits: efficacy of penicillin therapy. Lab Anim Sci. 1981;31:382–385. [PubMed] [Google Scholar]
- 19.Lu Y S, Pakes S P, Massey L. Hyperimmune serum from rabbits immunized with potassium thiocyanate extract of Pasteurella multocida protects against homologous challenge. J Clin Microbiol. 1987;25:2173–2180. doi: 10.1128/jcm.25.11.2173-2180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y S, Pakes S P, Massey L, Stefanu C. A potassium thiocyanate extract vaccine prepared from Pasteurella multocida 3:A protects rabbits against homologous challenge. Infect Immun. 1987;55:2967–2976. doi: 10.1128/iai.55.12.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 22.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 23.Mukkur T K S, Pyliotis N A. The potassium thiocyanate extract of Pasteurella multocida: electron microscopy and susceptibility of its immunogenic activity to some physical chemical and enzymatic treatments. J Comp Pathol. 1981;91:427–437. doi: 10.1016/0021-9975(81)90013-x. [DOI] [PubMed] [Google Scholar]
- 24.Petersen S K, Foged N T, Bording A, Nielsen J P, Riemann H K, Frandsen P L. Recombinant derivatives of Pasteurella multocida toxin: candidates for a vaccine against progressive atrophic rhinitis. Infect Immun. 1991;59:1387–1393. doi: 10.1128/iai.59.4.1387-1393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringler D H, Peter G K, Chrisp C E, Keren D F. Protection of rabbits against experimental pasteurellosis by vaccination with a potassium thiocyanate extract of Pasteurella multocida. Infect Immun. 1985;49:498–504. doi: 10.1128/iai.49.3.498-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahin R, Barbic J, Leef M. Proceedings of Mucosal Immunity: Cellular and Molecular Cross-Talk at Mucosal Surfaces. Santa Fe, N.Mex. 1997. IFN-γ and specific immunity in natural clearance of Bordetella pertussis infection (abstract) [Google Scholar]
- 27.Snipes K P, Hirsh D C, Kasten R W, Carpenter T E, Hird D W, McCapes R H. Differentiation of field isolates of Pasteurella multocida serotype 3,4 from live vaccine strain by genotypic characterization. Avian Dis. 1990;34:419–424. [PubMed] [Google Scholar]
- 28.Suckow M A, Bowersock T L, Nielsen K, Chrisp C E, Frandsen P L, Janovitz E B. Protective immunity to Pasteurella multocida heat-labile toxin by intranasal immunization in rabbits. Lab Anim Sci. 1995;45:526–532. [PubMed] [Google Scholar]
- 29.Suckow M A, Bowersock T L, Nielsen K, Grigdesby C F. Enhancement of respiratory immunity to Pasteurella multocida by cholera toxin in rabbits. Lab Anim. 1996;30:120–126. doi: 10.1258/002367796780865808. [DOI] [PubMed] [Google Scholar]
- 30.Taylor R N, Huong A Y, Fulford K M, Przybyszewski V A, Hearn T L. Quality control for immunologic tests. Atlanta, Ga: Centers for Disease Control; 1979. pp. 55–85. [Google Scholar]
- 31.Watts J L, Yancey R J, Jr, Salmon S A, Case C A. A 4-year survey of antimicrobial susceptibility trends for isolates from cattle with bovine respiratory disease in North America. J Clin Microbiol. 1994;32:725–731. doi: 10.1128/jcm.32.3.725-731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu H Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]