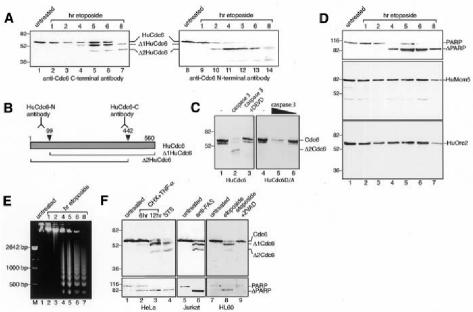
Fig. 1. HuCdc6 is cleaved in cells induced to undergo apoptosis. (A) Western blotting of nuclear extracts from HL60 cells treated with etoposide for the times indicated. An anti-C-terminal HuCdc6 antibody (lanes 1–7) and an anti-N-terminal HuCdc6 antibody (lanes 8–14) were used. (B) Diagram showing HuCdc6 and HuCdc6 fragments generated during apoptosis and the regions of HuCdc6 recognized by the antibodies used in this study. (C) Wild-type HuCdc6 and the caspase-resistant mutant HuCdc6D/A were transcribed and translated in vitro and incubated with caspase 3 (400 ng, left panel; 400 and 100 ng, right panel) or without (–). (D) PARP, HuMcm5 and HuOrc2 detected by western blotting of the same nuclear extracts as described above. Since HuMcm5 remains intact and its protein levels unchanged during apoptosis (Schwab et al., 1998), it is also used as a loading control for experiments in (A) and (D). (E) Analysis of genomic DNA fragmentation in HL60 cells by gel electrophoresis. (F) Western blotting of nuclear extracts from HeLa cells (induced to undergo apoptosis by either TNF-α plus CHX, lanes 2 and 3, or by STS, lane 4) and Jurkat cells (treated with anti-Fas antibody, lane 6). In vivo inhibition of caspases by the caspase-family inhibitor ZVAD–FMK is in lane 9. HuCdc6 was detected by using an anti-C-terminal HuCdc6 antibody. Bottom panels show cleavage of PARP.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.