Figure 1.
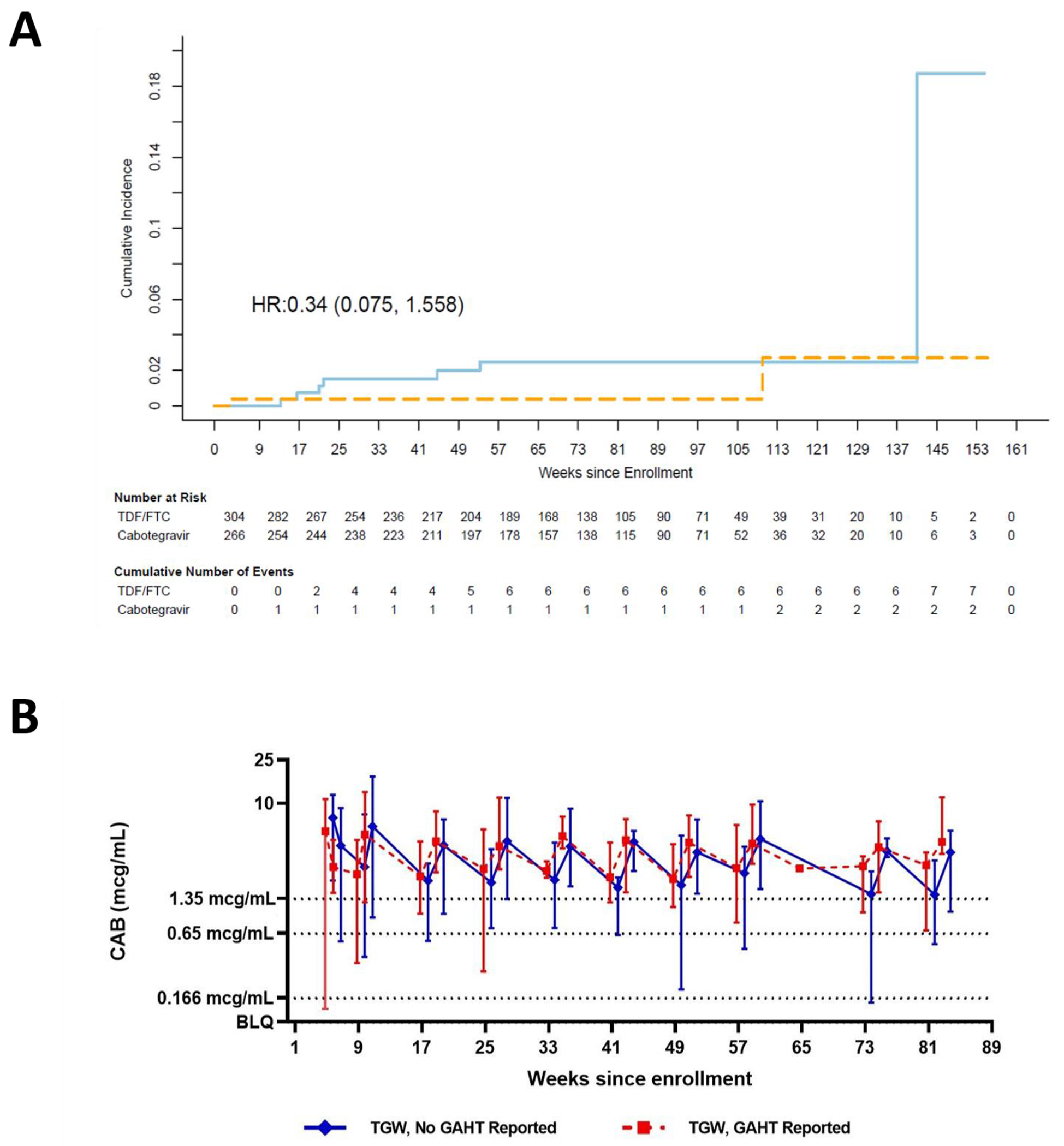
(A) Kaplan-Meier estimates of cumulative incidence of HIV infection among TGW in Intent to Treat Cohort. Solid light-blue line: TDF/FTC arm; Dashed orange line: Cabotegravir arm. TGW: transgender women, HR: Hazard ratio, TDF/FTC: tenofovir disoproxil fumarate/emtricitabine. (B) Median CAB concentrations in TGW in the absence (solid blue line) or presence (dotted red line) of GAHT. Error bars show the lower 5% and upper 95% of CAB concentrations observed at each time point. Dotted horizontal line: 1x PA-IC90 (0.166 μg/mL); dashed horizontal line: 4x PA-IC90 (0.664 μg/mL); solid horizontal line: 8x PA-IC90 (1.33 μg/mL). CAB: cabotegravir; GAHT: gender-affirming hormone therapy; PA-IC90: in vitro protein-adjusted 90% CAB inhibitory concentration.
