Table 2 |.
Asymmetric cyclopropanation of alkenes by [Fe(P3)Cl] with in situ-generated α-trifluoromethyldiazomethane and various diazo compounds
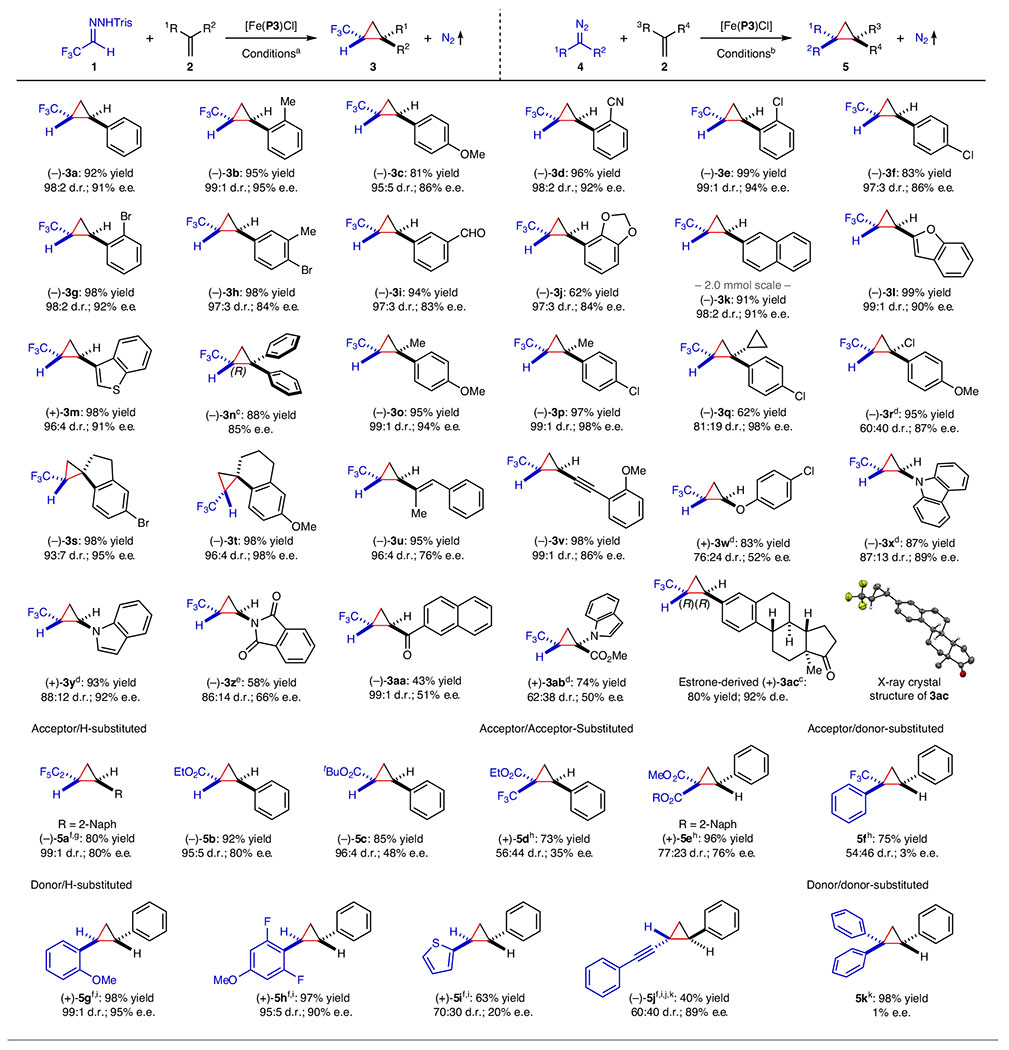
|
Conducted with hydrazone 1 (0.12 mmol) and alkene 2 (0.10 mmol) in the presence of Cs2CO3 (0.24 mmol) using [Fe(P3)Cl] as catalyst (2 mol%) in hexanes at 4 °C for 20 h; isolated yields; diastereomeric ratio (d.r.) determined by 19F NMR analysis of reaction mixture; enantiomeric excess (e.e.) of trans-isomer determined by chiral HPLC.
Conducted with diazo compound 4 (0.10 mmol) and alkene 2 (0.60 mmol) using [Fe(P3)Cl] as catalyst (2 mol%) in toluene at 23 °C for 24 h; isolated yields; d.r. determined by 1H NMR analysis of the reaction mixture; e.e. of the major isomer determined by chiral HPLC.
Absolute configuration determined by X-ray crystallography.
In toluene at 23 °C.
With 1 (0.20 mmol).
Diazo compound in situ-generated from trisylhydrazone in the presence of Cs2CO3 (0.20 mmol).
With 2 (0.12 mmol) in hexanes at 4 °C.
At 80 °C.
With 2 (0.20 mmol).
In ethyl acetate.
At 40 °C.
