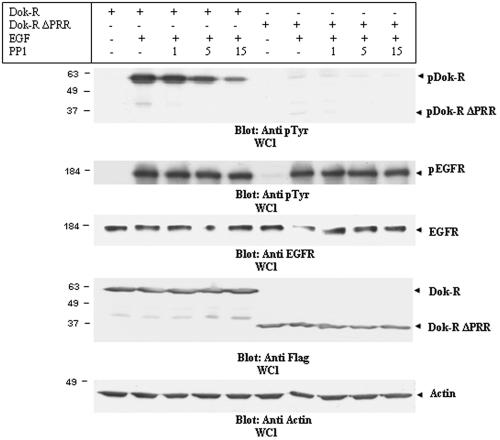
FIG. 5.
Dok-R is a substrate of SFKs. Cos1 cells transfected with either Dok-R or Dok-R ΔPRR were serum starved for 16 h prior to a two-hour pretreatment with PP1 (1, 5, and 15 μM) or vehicle (−). Following the pretreatment, cells were either left unstimulated (−) or stimulated (+) for 5 min with 100 ng/ml EGF. Cleared lysates were resolved via SDS-PAGE. Immunoblot analysis of lysates with an anti-pY antibody demonstrates that Dok-R and, to a much lesser degree, Dok-R ΔPRR become tyrosine phosphorylated in response to EGF stimulation. Increasing concentrations of PP1 are able to completely abolish phosphorylation of Dok-R ΔPRR, while phosphorylation of Dok-R is severely impaired in a dose-dependent manner by PP1 treatment. Changes in Dok-R phosphorylation are not a reflection of changes in total Dok-R or Dok-R ΔPRR protein levels (anti-HA, WCL), nor are they due to PP1-dependent changes in EGFR activation (anti-pY EGFR) or EGFR levels (anti-EGFR).