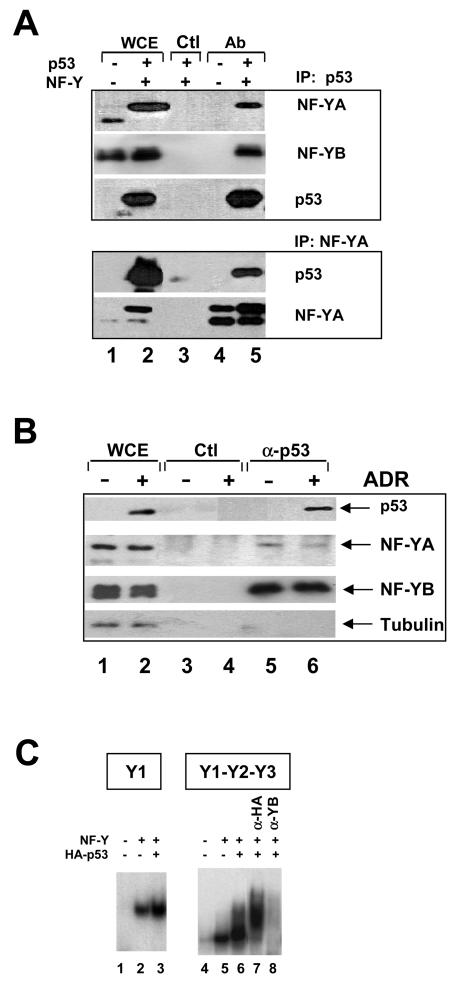
FIG. 4.
Binding of p53 to NF-Y in vivo. (A) NF-Y-p53 interactions in vivo in H1299 cells. Immunoprecipitation (IP) with anti-p53 or anti-YA (lanes 4 and 5), and control (Ctl) (lane 3) antibodies (Ab) was followed by Western blot analysis with the indicated antibodies. In lanes 1 and 2, extracts were tested directly in Western blots. NF-Y and p53 were overexpressed in the extracts used in lanes 2, 3, and 5. The different sizes of NF-YA are due to the prevalence of the “short” splicing isoform in lane 1 and overexpression of the “long” isoform in lanes 2, 3, and 5. WCE, whole-cell extracts. (B) Evaluation of endogenous NF-Y-p53 interactions. NIH 3T3 cells were not treated (lanes 1, 3, and 5) or were treated with adriamycin (ADR) for 8 h (lanes 2, 4, and 6). Extracts were analyzed directly in Western blots (lanes 1 and 2) or immunoprecipitated with control (lanes 3 and 4) or anti-p53 (lanes 5 and 6) antibodies. Western blot analysis of eluates with the indicated antibodies is shown in lanes 3 to 6. (C) EMSAs of in vivo-produced NF-Y and p53. The Y1 CCAAT oligonucleotide was used in lanes 1 to 3; a cyclin B2 fragment (−129 to +48) was used in lanes 4 to 8. In lanes 1 and 4, 1 μl of mock-transfected Saos2 cell extracts was used; in lanes 2, 3, and 5 to 8, equivalent amounts of extracts from cells transfected with NF-Y expression vectors were used, together with HA-p53 in lanes 3 and 6 to 8. Supershift with anti-HA antibodies and inhibition with anti-YB antibodies are shown in lanes 7 and 8, respectively.