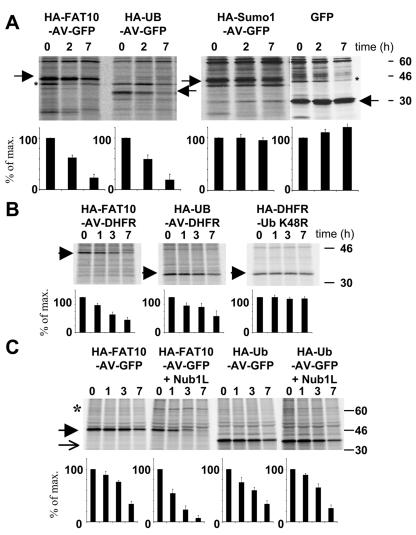
FIG. 2.
FAT10 and ubiquitin are equally potent in targeting for degradation. (A) FAT10, SUMO, and ubiquitin were transiently expressed as uncleavable AV/GG mutated and HA-tagged GFP fusion proteins in HeLa cells. The cells were labeled for 1 h with [35S]Cys-Met and chased for the indicated time periods prior to HA-specific immunoprecipitation, SDS-polyacrylamide gel electrophoresis (PAGE), autoradiography, and quantification on a radioimager. As a control, GFP was expressed and immunoprecipitated with an anti-GFP antibody. The arrowheads denote the different GFP fusion proteins and GFP as indicated above; an asterisk indicates an unspecific band. (B) Fusion proteins of DHFR with HA-tagged FAT10 and ubiquitin as well as a DHFR-HA-UbK48R control were expressed in HeLa cells, and a pulse-chase experiment was performed as shown for panel A. The arrowheads denote the respective DHFR fusion proteins as indicated above the panels. (C) HA-FAT10-GFP and HA-ubiquitin-GFP were transiently expressed in HeLa cells without or together with HA-NUB1L. A pulse-chase analysis was performed as shown for panels A and B. An arrow denotes HA-Ub-AV-GFP, and an arrowhead denotes HA-FAT10-AV-GFP. All bands were quantified on a radioimager and plotted below each lane as percent radioactivity based on values obtained after the pulse. The data represent means of results from three independent experiments ± standard errors of the means.