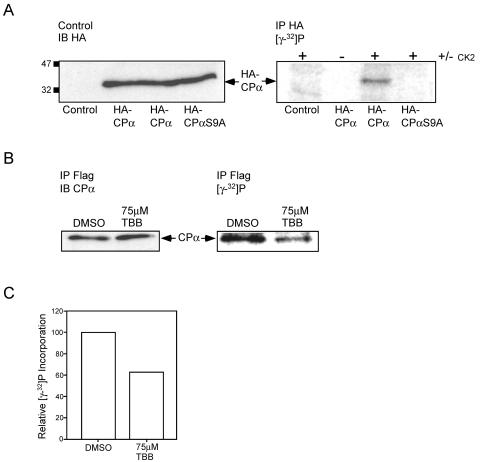
FIG. 8.
Phosphorylation of CPα by protein kinase CK2 in vitro and in cells. (A) Protein kinase CK2 phosphorylates CPα on serine 9 in vitro. HA-CPα, HA-CPαS9A, and empty vector were transiently transfected into U2-OS cells. (Left panel) Lysates derived from these cells were immunoblotted (IB) with biotinylated anti-HA antibodies to demonstrate equal loading. (Right panel) The lysates were subjected to immunoprecipitation (IP) with anti-HA antibodies. Bound proteins were phosphorylated with recombinant protein kinase CK2 on the protein A-Sepharose beads in the presence of [γ-32P]ATP, separated by SDS-PAGE, and visualized using a PhosphorImager. The position of HA-CPα is indicated. (B and C) Endogenous CPα is phosphorylated in cells, and treatment with a CK2 specific inhibitor results in a decrease in phosphate incorporation. (B) DC1.4 cells expressing Flag-CKIP-1 were grown in the presence of 75 μM TBB or carrier alone for 18 h and labeled with 0.33 mCi of [γ-32P]orthophosphate for 6 h. Duplicate plates were treated identically but in the absence of 0.33 mCi of [γ-32P]orthophosphate. Lysates derived from these cells were normalized for protein concentration, and subjected to immunoprecipitation with anti-Flag M2 antibodies. (Left panel) Immunoprecipitates from nonlabeled plates were transferred to PVDF membranes and immunoblotted with anti-CPα antibodies. (Right panel) Immunoprecipitates from labeled plates were separated by SDS-PAGE, dried, and exposed in a PhosphorImager to visualize labeled proteins. The position of CPα is indicated. (C) Data in panel B were analyzed using ImageQuant software and expressed as relative phosphate incorporation.