Figure 3. Conserved biological mechanism of thioredoxin.
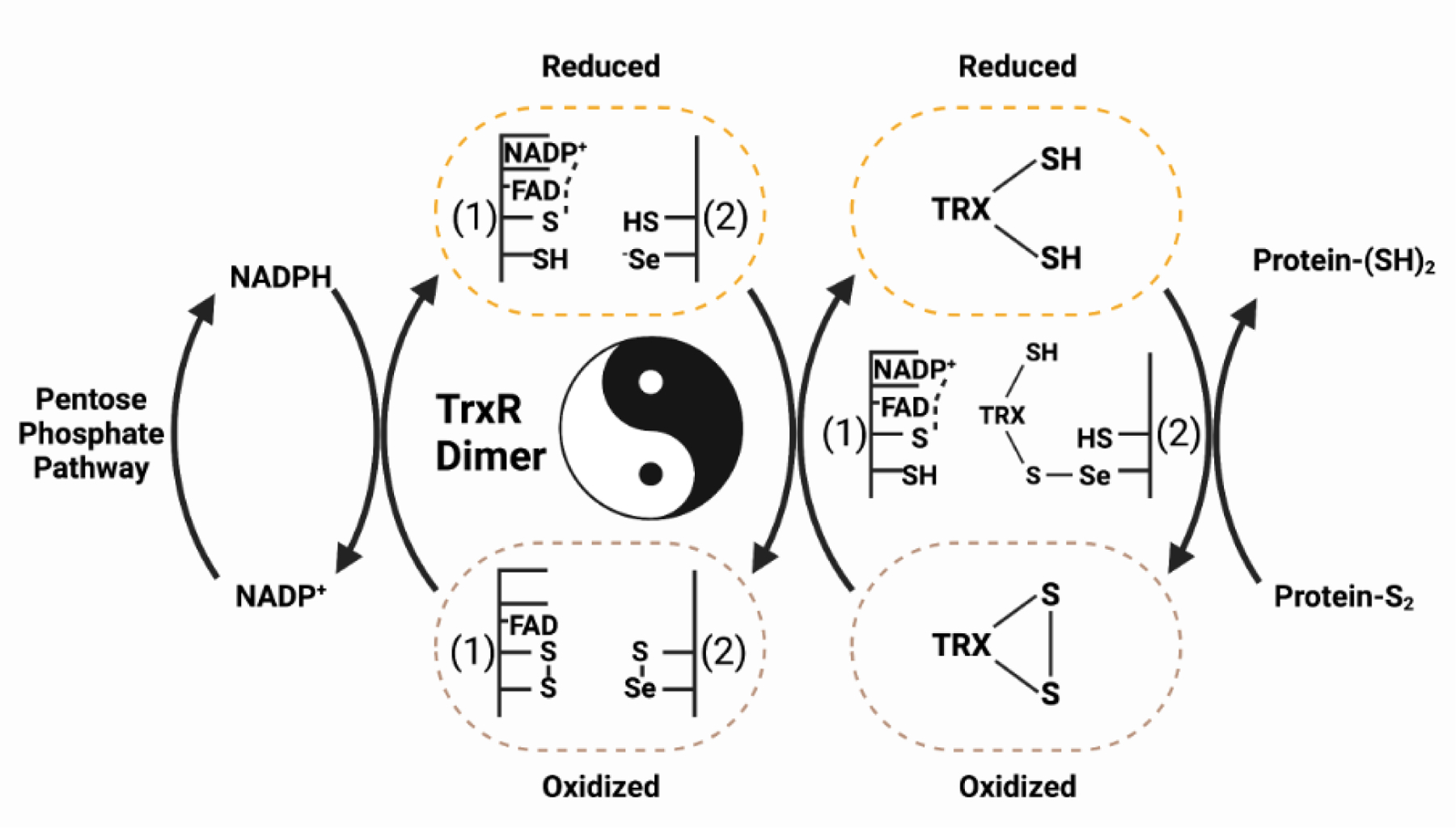
The thioredoxin pathway allows electrons from metabolism to cycle through the redox machinery, thereby maintaining a reduced cellular environment. From left to right, NADPH generated from the pentose phosphate metabolic pathway binds to a dimer of oxidized thioredoxin reductase (TrxR). Next, the TrxR dimer forms a yin-yang orientation where the “head” of protein 1 (1) binds into the “tail” of protein 2 (2) to reduce a Se-S bond mediated through an FAD cofactor. This process is performed in duplicate with the “tail” of (1) binding into the “head” of (2) (not shown). Third, the reduced TrxR dimer can then recycle oxidized thioredoxin by binding to the selenocysteine of the reduced TrxR. The resulting electron shuttle restores thioredoxin to its reduced form, thus regaining its cellular redox capabilities.
