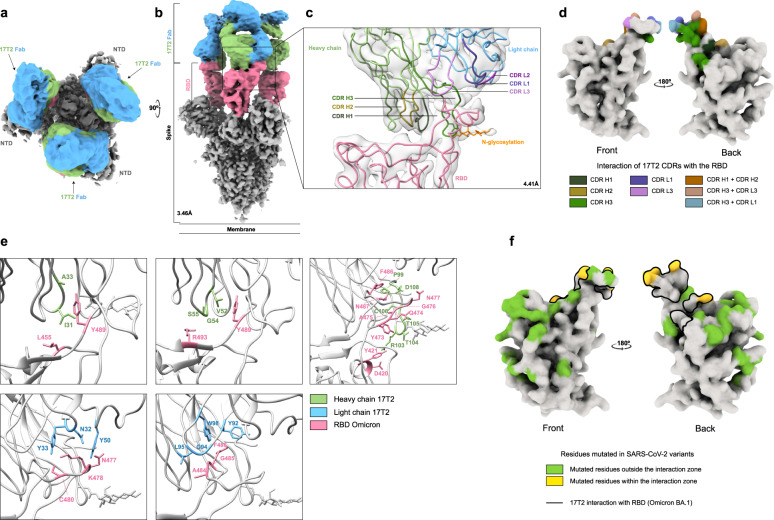
Fig. 4. Structural and functional characterization of complex 17T2 Fab fragment with Omicron BA.1 spike using cryo-EM.
a, b Top and side views of the cryo-EM map of Omicron BA.1 spike trimer with three 17T2 Fab fragments bound to three open RBDs, at 3.46 Å resolution. The core of the spike is shown in gray, the RBDs in pink, and the heavy chain (HC) and light chain (LC) of the 17T2 Fab in green and blue, respectively. c Structure of the RBD and 17T2 Fab after local refinement, at 4.41 Å resolution. The interaction zone between the RBD and the Fab is shown in cartoon representation where the three CDRs from each chain are distinctively colored and the N-glycosylation is indicated in orange. d CDRs that are involved in binding with RBD, specifically, interacting in the region of its left shoulder and neck (front and back view, respectively). e A detailed view of some of the residues involved in the interaction between 17T2 Fab and RBD. The main chains are colored in gray and the side chains of the residues involved in the interaction are shown in green for HC, in blue for LC, and in pink for RBD. f Locations of SARS-CoV-2 variant mutations on RBD relative to 17T2 epitope site that is shown as a black line (front and back view, respectively). The information about the variants and mutated residues can be found in Supplementary Table 4.