Abstract
Purpose
The objective of the COLLISION RELAPSE trial is to prove or disprove superiority of neoadjuvant systemic therapy followed by repeat local treatment (either thermal ablation and/or surgical resection), compared to repeat local treatment alone, in patients with at least one recurrent locally treatable CRLM within one year and no extrahepatic disease.
Methods
A total of 360 patients will be included in this phase III, multicentre randomized controlled trial. The primary endpoint is overall survival. Secondary endpoints are distant progression-free survival, local tumour progression-free survival analysed per patient and per tumour, systemic therapy-related toxicity, procedural morbidity and mortality, length of hospital stay, pain assessment and quality of life, cost-effectiveness ratio and quality-adjusted life years.
Discussion
If the addition of neoadjuvant systemic therapy to repeat local treatment of CRLM proves to be superior compared to repeat local treatment alone, this may lead to a prolonged life expectancy and increased disease-free survival at the cost of possible systemic therapy-related side effects.
Level of Evidence
Level 1, phase III randomized controlled trial.
Trial Registration
NCT05861505. May 17, 2023.
Graphical Abstract
Keywords: Colorectal cancer, Colorectal liver metastases (CRLM), Neoadjuvant systemic therapy, Thermal ablation, Surgery, Randomized controlled trial (RCT)
Introduction
Colorectal cancer (CRC) is a common type of cancer, with an incidence of nearly two million new cases and a mortality rate of more than 900,000 deaths per year worldwide [1]. The prognosis largely depends on the presence of distant metastases. Up to 50% of patients with CRC develop colorectal liver metastases (CRLM) [2, 3]. Without any treatment, the 5-year overall survival (OS) rate is below 3% and when systemic therapy is administered, 5-year OS reaches 11% [4–6]. Local treatment comprising partial hepatectomy and/or thermal ablation offers potential cure, with 5-year OS rates of 44–58% [3, 7–9]. Despite complete tumour eradication, approximately 64–85% of patients develop new metastases after the first local treatment of CRLM [10, 11].
To treat intrahepatic recurrences, partial hepatectomy and/or thermal ablation are considered standard of care in current literature and international guidelines [12–16]. After upfront repeat local treatment, 5-year OS is 51% [17–20]. Early recurrent CRLM (≤ 12 months) are associated with poorer prognosis due to presumed worse tumour biology and the presence of intrahepatic micrometastases [21]. Heise et al. reported inferior disease-free survival (DFS) after repeat local treatment compared to the initial local treatment (p < 0.001) [22]. Therefore, neoadjuvant systemic therapy prior to repeat local treatment has been suggested to prolong both OS and DFS [23]. A recent pooled meta-analysis showed a trend towards improved survival with the addition of neoadjuvant systemic therapy to repeat local treatment [24]. Nevertheless, the side effects and toxicity of systemic therapy and impact on quality of life (QoL) should be carefully considered [25, 26].
The value of neoadjuvant systemic therapy prior to repeat local treatment in case of recurrent and locally treatable CRLM remains uncertain [24]. To assess the impact of neoadjuvant systemic therapy, we have designed a phase III randomized controlled trial (RCT) directly comparing upfront local treatment (control group) with neoadjuvant systemic therapy followed by local treatment (intervention group) in patients with recurrent CRLM within 12 months after initial local treatment.
Design
Design
The COLLISION RELAPSE trial is a phase III, multicentre randomized controlled trial initiated by the Amsterdam University Medical Centers (Amsterdam UMC), location VUmc in Amsterdam, the Netherlands. This study is endorsed by the Dutch Colorectal Cancer Group (DCCG) and by the Dutch Colorectal Cancer Foundation. Patients will be recruited in at least four centres in the Netherlands: Amsterdam UMC (location VUmc); Noordwest Ziekenhuisgroep Alkmaar; Leiden University Medical Center and Máxima Medisch Centrum, Veldhoven and Eindhoven. Additional Dutch and Belgian high volume liver centres are expected to contribute pending local approvals.
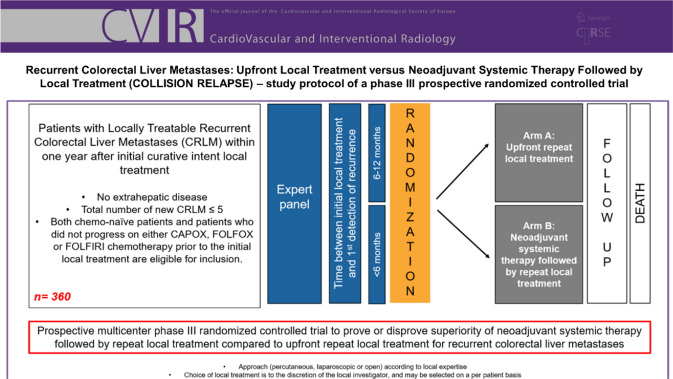
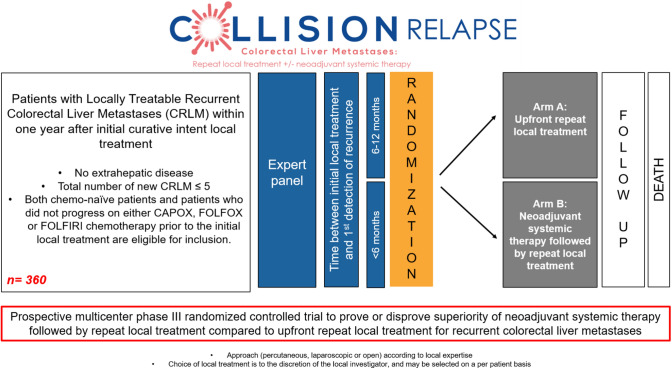
The trial is investigator-initiated, independent of industry and registered at clinicaltrials.gov under number NCT05861505. The protocol has been approved by the Amsterdam UMC, Medical Ethical Review Board (METc; no. 2022.0093—NL78220.029.21). The trial will be conducted in accordance with the Declaration of Helsinki and the guidelines for Good Clinical Practice (GCP). The flow chart of the study design is shown in Fig. 1. Inclusion, randomization and treatments started from April 2023 in four hospital centres.
Fig. 1.
Flow chart of the study design
Inclusion Criteria
Patients with ≥ 1 locally treatable recurrent CRLM within 12 months following first local treatment, no microsatellite instability (MSI), no extrahepatic disease, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and/or an American Society of Anesthesiologists (ASA) status of 1–3 are considered eligible (Table 1). Partial hepatectomy and/or thermal ablation is allowed with a maximum number of 5 recurrent CRLM.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age > 18 years | Extrahepatic disease |
| Performance status (ECOG 0–2 or ASA 1–3) | MSI/dMMR |
| Histological documentation of primary colorectal tumour (adenocarcinoma) | Radical local treatment unfeasible or unsafe (e.g. insufficient future liver volume) |
| Local treatment performed for initial CRLM | Compromised liver function (e.g. signs of portal hypertension, INR > 1.5 without use of anticoagulants, ascites) |
| New recurrence ≤ 12 months | Uncontrolled infections (> grade 2 NCI-CTC version 3.0) |
| ≥ 1 locally treatable CRLM (resectable* and/or ablatable) | Pregnant or breast-feeding subjects |
| Total number of new CRLM ≤ 5 | Immuno- or chemotherapy ≤ 6 weeks prior to the randomization |
| Chemo-naïve or history of response to CAPOX/FOLFOX/FOLRIRI | Severe allergy to contrast media not controlled with premedication |
| Life expectancy of at least 12 weeks | Substance abuse, medical, psychological or social conditions that may interfere with the subject’s participation in the study or evaluation of the study results |
|
Adequate bone marrow, liver and renal function: Haemoglobin ≥ 5.6 mmol/L Absolute neutrophil count (ANC) ≥ 1,500/mm3 Platelet count ≥ 100*109/l Total bilirubin ≤ 1.5 times the upper limit of normal ALT and AST ≤ 2.5 × upper limit of normal (≤ 5 × upper limit of normal for subjects with liver involvement of their cancer) Albumin > 30 g/l Serum creatinine ≤ 1.5 × upper limit of normal or a MDRD ≥ 50 ml/min Prothrombin time or INR < 1.5 × ULN, unless coumarin derivates are used. Due to interactions with capecitabine, all patients using coumarin derivates will be treated with LMWH instead Activated partial thromboplastin time < 1.25 × ULN (therapeutic anticoagulation therapy is allowed if this treatment can be interrupted as judged by the treating physician) |
|
| Written informed consent |
ECOG Eastern Cooperative Oncology Group, ASA American Society of Anesthesiologists, MSI Microsatellite instability, dMMR deficient mismatch repair
*Resection for resectable lesions considered possible by obtaining negative resection margins (R0) and preserving adequate liver reserve
Exclusion Criteria
Patients with expert panel ineligibility prior to randomization will not be included. Recruited (included and randomized) patients are considered drop-outs when lost to follow-up or if patients actively decide to withdraw from the study at any time for any reason. Within the study protocol, crossover between treatment arms is not allowed. Patients in the intervention group will be excluded from the study if they refuse to start with neoadjuvant systemic therapy for any reason.
Regardless of the treatment arm, patients with previously undetected disease (e.g. detection of peritoneal metastases during surgery) or progressive remain study participants according to the intention-to-treat study design.
Statistics
We hypothesize that neoadjuvant systemic therapy prior to repeat local treatment (either thermal ablation and/or surgical resection) is superior to direct local treatment for the selected patient groups in terms of the primary objective (overall survival). The Cox proportional hazards model (1-sided; superiority) is used for the sample size calculations. We consider OS improved if increased by 5% with 3-year follow-up with corresponding hazard ratio (HR) of 0.7 to represent the upper limit of superiority, based on guidelines provided by the Dutch Society of Medical Oncology [27]. The total number of events needed to estimate the HR of 0.7 with 80% at a significance level (alpha) of 0.05 is equal to 195. Following results of the LiverMetSurvey by Viganó et al. [28], 5-year probability of event is 55.9% (overall probability of event, pE = 0.559). Therefore, the calculated raw sample size is 348 (NRS). A 3% drop-out rate due to loss to follow-up or if patients actively decide to withdraw from the study at any time for any reason, is taken into account (NDO = 12). A total number of 360 patients will be randomized (NR) into: arm A (control group) upfront repeat local treatment (n = 180) and arm B (intervention group) 12 weeks of neoadjuvant systemic therapy followed by repeat local treatment (n = 180).
All basic patient, procedure and tumour-related characteristics will be summarized and evaluated with standard descriptive statistics. Categorical variables will be tabulated with number of patients and analysed between arms using Fisher’s exact test and Pearson Chi square test. Continuous variables will be reported as means, standard deviations, medians and (interquartile) ranges. All p values below 0.05 will be considered significant.
The primary endpoint OS and secondary endpoints DPFS and LTPFS per patient and per tumour are defined as time-to-event from randomization and local treatment, respectively, and analysed using Kaplan–Meier curves with the log-rank test. In addition, Cox proportional hazards regression models are used to perform univariable and multivariable analysis on basic patient, procedure and tumour-related characteristics. Systemic therapy-related toxicity and procedural morbidity and mortality will be described using Common Terminology Criteria for Adverse Events 5.0 (CTCAE) [29] and compared between arms using Pearson Chi square test. The length of hospital stay will be assessed using Mann–Whitney U Test. Visual analogue scale questionnaires will be used to assess pain prior to, directly after and every three months after repeat local treatment and compared using linear mixed models. Quality of life questionnaires will be conducted prior to, and every three months after repeat local treatment, and will also be assessed prior to, during and after neoadjuvant systemic therapy. Differences between arms will be evaluated with linear mixed models. Incremental Cost-effectiveness Ratio (ICER) will be calculated with direct and indirect total cost care for both arms and used to perform a cost-utility analysis using Quality-adjusted Life Years (QALY) to calculate years of full health lived.
Statistical analyses will be conducted using SPSS® Version 28.0 (IBM®, Armonk, New York, USA) [30] and R version 4.0.3. (R Foundation, Vienna, Austria) [31]. The statistical plan is supported by a biostatistician (BLW).
Study Cohort
All patients will be discussed in the local multidisciplinary liver tumour boards of the participating centre. Potential candidates will be registered and undergo a routine, national guideline-based pre-procedural work-up: contrast enhanced (ce)CT of the chest and abdomen, ceMRI including diffusion-weighted imaging (DWI), 18F-FDG PET-CT, anaesthetic review, baseline full blood examination, urea and electrolytes, renal function tests, liver enzymes and coagulation profile test.
The patient’s medical history and cross-sectional imaging will be reviewed for eligibility by an expert panel consisting of at least two abdominal radiologists, two interventional radiologists, two hepatobiliary surgeons and two medical oncologists. Patients will provide consent for multidisciplinary peer consultation. After referral of potential eligible patients from the tumour board, the expert panel then confirms inclusion and arranges randomization if these patients are indeed locally treatable (either by thermal ablation and/or surgical resection). A small proportion of patients are likely to be rejected by the expert panel. After confirmation by the expert panel, informed consent will be obtained and the patients will be randomized. In addition, cross-sectional imaging of all patients receiving neoadjuvant systemic therapy are re-reviewed by the expert panel after completing all cycles in this trial to detect possible changes in treatment plan and chemotherapeutical efficacy.
After written informed consent is obtained, patients will be included and randomized. Patients should be scheduled to start systemic therapy or undergo repeat local treatment within a period of six weeks following randomization. Choice of repeat local treatment is at the discretion of the local investigator in consultation with local multidisciplinary liver tumour boards of the participating centre. Upfront repeat partial hepatectomy and/or thermal ablation should be scheduled according to the national guidelines [32], at least 4 weeks and at most 12 weeks after the last cycle of systemic therapy.
Randomization
Patients will be randomized into: arm A (upfront repeat local treatment) and arm B (neoadjuvant systemic therapy followed by repeat local treatment). Randomization is centralized and performed using Castor EDC (electronic data capture). Eligible patients will be stratified according to: the interval between initial local treatment and first detection of recurrent CRLM (≤ 6 months vs. 6–12 months), RAS mutations vs RAS wildtype, BRAF mutation vs BRAF wildtype, prognostic risk score (modified clinical risk score Brudvik et al. [33], low vs. high risk) and previous systemic therapy versus no previous systemic therapy.
Partial Hepatectomy
Partial hepatectomy will be conducted under general anaesthesia during laparoscopy or open laparotomy, based on the judgement of the surgeon performing the procedure. The abdominal cavity will be explored in order to exclude extrahepatic tumour manifestations by an experienced hepatobiliary surgeon, i.e. a certified oncological surgeon with broad expertise (having performed and/or supervised > 100 liver tumour resection procedures).
The surgeon will remove all target lesions whether or not combined with thermal ablation performed by an interventional radiologist. The extent of the resection, the resection margins and the specific technique is at the discretion of the performing liver surgeon (but should have the intention and thus the preoperative estimation of a possible pathological R0 resection, while preserving a sufficient future liver remnant). Postoperative care will be on the recovery ward and subsequently on either the surgery ward or medium care whenever deemed necessary.
Thermal Ablation
The interventional radiologist will ablate all target lesions whether or not combined with partial hepatectomy performed by a surgeon. Patients without contra-indications for a percutaneous approach will undergo percutaneous thermal ablation. Contra-indications for a percutaneous approach are proximity of critical structures. To avoid collateral damage to intestines a minimum distance to the stomach, small bowel and colon of 15 mm should be respected. Therefore, pneumo- or hydro-dissections are allowed. To assess technical efficacy, a ceCT or ceMRI should be performed at the time of the procedure. Patients with a contra-indication for a percutaneous approach will undergo open laparoscopic or ablation.
Thermal ablation (either radiofrequency ablation, RFA; or microwave ablation, MWA) is performed according to the CIRSE quality improvement guidelines with an intentional tumour-free ablation margin of at least 1 cm by an experienced operator, i.e. having performed and/or supervised > 100 thermal ablation procedures. The definition of a technically successful ablation is based upon the specific protocols established by the device manufacturers in combination with immediate post procedurally performed ultrasound in case of open approaches (fully hyperechoic ablation zone with an intentional margin of at least 1 cm) or imaging with confirmation software in case of percutaneous approaches. Necessity for re-ablations during the procedure (completion of the procedure) and/or needle repositioning will be judged by the performing interventional radiologist. Postoperative care will be on the recovery and subsequently on either the surgery ward or medium care whenever deemed necessary.
Neoadjuvant Systemic Therapy
In case of randomization to arm B, patients receive maximum 12 weeks (4/6 cycles) of neoadjuvant systemic therapy. In case of delayed delivery of planned systemic therapy, the treatment will be maintained for a maximum duration of 12 weeks. Patients receive 4 cycles of CAPOX (capecitabine with oxaliplatin) or 6 cycles of FOLFOX/FOLFIRI (5-fluorouracil/leucovorin with either oxaliplatin or irinotecan), both with or without bevacizumab. The choice of agent is regardless of the location of the primary tumour, RAS mutation status, BRAF mutation status and previously received systemic therapy following primary tumour resection or previously received induction chemotherapy for initial downstaging of CRLM. The choice of treatment is at the discretion of the local medical oncologist.
A baseline ceCT or 18F-FDG-PET-CT will be performed no more than 28 days prior to the first dose of chemotherapeutic treatment. After 3 cycles of CAPOX or 4 cycles of FOLFOX/FOLFIRI (with or without bevacizumab), a follow-up ceCT will be acquired and response rates will be evaluated according to Response Evaluation Criteria in Solid Tumours (RECIST guideline, version 1.1) [34]. Patients who show clinical benefit, defined as stable disease or response to therapy, will be treated with additional 1 (CAPOX) or 2 (FOLFOX/FOLFIRI) cycle(s) of neoadjuvant systemic therapy without bevacizumab, followed by repeat local treatment. Patients with disease progression who, based on ceMRI, still qualify for repeat local treatment according to the expert panel, will receive repeat local treatment within 4–12 weeks. CRLMs in complete remission, confirmed by contrast enhanced magnetic resonance imaging (ceMRI), will not be locally treated but will be monitored using cross-sectional imaging. Patients with progressive disease will be treated according to best clinical practice, including all treatment modalities and remain in the trial group according to intention-to-treat. Supportive care for treatment-related symptoms will be offered as needed to all patients in this study.
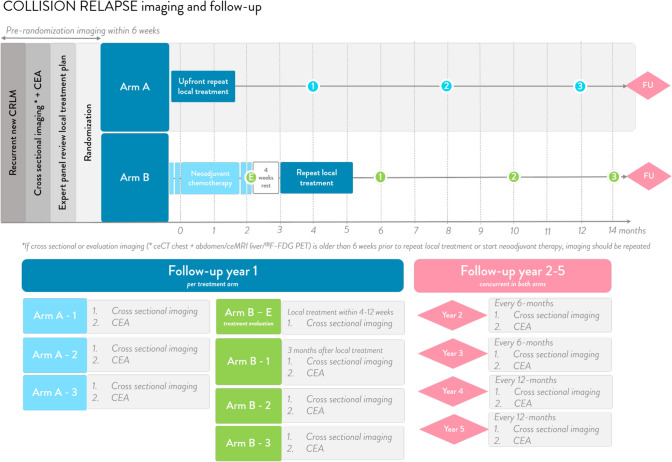
Follow-up
The follow-up scheme is presented in Fig. 2 and based on (inter)national standards. Patients with disease progression will be treated according to best clinical practice, including all treatment modalities and remain in the trial group according to intention-to-treat.
Fig. 2.
Follow-up scheme
Outcome Measures
The main objective is to prove or disprove superiority of neoadjuvant systemic therapy followed by repeat local treatment compared to upfront repeat local treatment in patients with at least one locally treatable recurrent CRLM and no extrahepatic disease. The primary endpoint is overall survival (OS). Secondary endpoints are distant progression-free survival (DPFS), local tumour progression-free survival (LTPFS) per patient and per tumour, systemic therapy-related toxicity, procedural morbidity and mortality, length of hospital stay, pain assessment and QoL, cost-effectiveness ratio (ICER) and quality-adjusted life years (QALY).
Data Monitoring
An independent monitor committee (Clinical Research Bureau) will maintain the quality of this investigator-initiated study according to GCP. Onsite monitoring including source data verification, to verify similarity between data on the case report form and source data, will be performed. In addition, all informed consent forms, inclusion and exclusion criteria and primary outcome OS will be confirmed for all participants.
Serious Adverse Events
Lastly, all serious adverse events (SAE) will be reported to ToetsingOnline and the METc, after which the reports are checked on adequacy and compliance with legal rules and regulations. All SAE’s, both related and unrelated to the treatment will be reported within 15 days after notification, or within 7 days if the SAE is life-threatening or resulted in death.
Discussion
Literature to support repeat local treatment of recurrent CRLM is well established. Multiple studies have shown a superior OS and DFS of repeat local treatment over palliative chemotherapy alone, resulting in a high number of long-term survivors [17–19]. Studying a selected group of patients receiving second local treatment, international large retrospective series show 5-year OS rates of nearly 51%, without compromising safety [17–19, 35]. International guidelines recommend either repeat partial hepatectomy and/or thermal ablation to treat recurrent CRLM, unless patients are not fit for local treatment or further treatment is not considered beneficial, then systemic therapy or palliative care is preferred [12–14, 36, 37]. For patients with recurrent disease after first local treatment, immediate repeated local treatment is considered to be the standard of care [12–15].
The EORTC 40983 trial by Nordlinger et al. and the JCOG 0603 trial by Kanemitsu et al. showed no benefit with the addition of (neo)adjuvant systemic therapy of resectable and/or ablatable disease after first local treatment of CRLM [38, 39]. Therefore, the role of systemic therapy remains reserved for limited purposes. For example, in to downstage CRLM to resectable and/or ablatable disease or to reduce procedural risks [40, 41]. However, the largest to date registry study (LiverMetSurvey) found an OS benefit favouring the use of neoadjuvant systemic therapy before repeat local treatment: 5-year OS: 61.5% versus 43.7% (HR = 0.529; p = 0.028) [28]. The authors advocate the use of neoadjuvant systemic therapy to adequately select good candidates for repeat local treatment and to control rapidly progressive disease. A recent pooled meta-analysis showed a trend towards improved survival, but the results remain indecisive and no conclusions could be drawn to define the role of neoadjuvant systemic therapy in recurrent CRLM [24].
Furthermore, recurrent disease is associated with micrometastatic disease and dormant cancer cells, which are not addressed by repeat local treatment alone [21]. This potentially indicates a higher risk profile, where providing aggressive systemic as well as local treatment is suggested[42, 43]. Moreover, the use of neoadjuvant systemic therapy may improve selection of patients eligible for repeat local treatment by adjusting treatment strategy to tumour biology and it may decrease risks of repeat local treatment [44–47]. If tumour shrinkage is observed during the administration of neoadjuvant systemic, studies suggested an increased rate of complete resection rates [44]. No (inter)national guideline organizations and scientific societies clearly discuss the position of neoadjuvant systemic therapy in recurrent CRLM.
Besides the potential benefits, the well-known risks and toxicities of systemic therapy should be taken into account [25, 26]. In addition, during the administration of neoadjuvant systematic therapy in recurrent CRLM, high rates of chemotherapeutic side effects and complications (46.7%) and lower QoL were found [48]. Other retrospective series did not report systematic therapy-related impact on repeat local treatment nor detected any increase in periprocedural complications or length of hospital [49, 50]. Neoadjuvant systemic therapy is specifically found to be safe if patients are not overtreated before surgical resection or thermal ablation [21]. No negative effect on periprocedural morbidity or liver function was found, when adhering to a maximum of 12 weeks of neoadjuvant systemic therapy [51–57].
In conclusion, neoadjuvant systemic therapy prior to repeat local treatment has been suggested to prolong survival, to eliminate micrometastatic disease, to eradicate dormant cancer cells in the liver, to decrease the risk of recurrences and to control rapidly progressive disease. However, the role of neoadjuvant systemic therapy in recurrent new and locally treatable CRLM remains uncertain. To assess the added value of neoadjuvant systemic therapy, we have designed a phase III randomized controlled trial (RCT) directly comparing upfront repeat local treatment (control) with neoadjuvant systemic therapy followed by repeat local treatment (intervention).
Acknowledgements
The authors thank all the COLLISION trial group members for their collaboration in local participating centres.
Abbreviations
- (S)AE
(Serious) Adverse Events
- 18F-FDG
[18F]-fluoro-2-deoxy-d-glucose
- ASA
American Society of Anesthesiologists
- BRAF
V-raf Murine Sarcoma Viral Oncogene Homolog B
- CAPOX
Capecitabine with oxaliplatin
- Ce
Contrast-enhancement
- CEA
Carcinoembryonic Antigen
- CRC
Colorectal Cancer
- CRLM
Colorectal Liver Metastases
- CT
Computed Tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DCCG
Dutch Colorectal Cancer Group
- DPFS
Distant Progression-free Survival
- DWI
Diffusion-weighted Imaging
- ECOG
Eastern Cooperative Oncology Group
- FOLFIRI
5-Fluorouracil/leucovorin with irinotecan
- FOLFOX
5-Fluorouracil/leucovorin with oxaliplatin
- GCP
Good Clinical Practice
- ICER
Cost-Effectiveness Ratio
- LTPFS
Local Tumour Progression-free Survival
- METc
Medical Ethical Review Board
- MRI
Magnetic Resonance Imaging
- MSI
Microsatellite Instability
- OS
Overall Survival
- PET
Positron Emission Tomography
- QALY
Quality-adjusted Life Years
- QoL
Quality of Life
- RAS
Rat Sarcoma Viral Oncogene Homolog
- RCT
Randomized Controlled Trial
Funding
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for Publication
For this protocol consent for publication is not required.
Ethics Approval and Consent to Participate
Obtained by Medisch Ethische Toetsingscommissie (METc) Amsterdam UMC. Reference number 2022.0093—NL-number NL78220.029.21.
Human and Animal Participants
All procedures performed in studies involving human participants will be in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent will be obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Madelon Dijkstra and Babette I. Kuiper: Shared first authorship.
Kathelijn S. Versteeg and Martijn R. Meijerink: Shared senior authorship.
References
- 1.WHO. Estimated age-standardized incidence rates (World) in 2020, all cancers, both sexes, all ages 2020. http://gco.iarc.fr/today/online-analysis-map.
- 2.Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meijerink MR, Puijk RS, van Tilborg A, Henningsen KH, Fernandez LG, Neyt M, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204. doi: 10.1007/s00270-018-1959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77(11):1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 5.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–1410. doi: 10.1016/S0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Liao F, Huang Y, Jiang C, Liu S, He W, et al. Longterm effects of palliative local treatment of incurable metastatic lesions in colorectal cancer patients. Oncotarget. 2016;7(15):21034–21045. doi: 10.18632/oncotarget.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984;199(5):502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143(12):1204–1212. doi: 10.1001/archsurg.143.12.1204. [DOI] [PubMed] [Google Scholar]
- 9.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiura A, Yamamoto J, Hasegawa K, Koga R, Sakamoto Y, Hata S, et al. Liver resection for multiple colorectal liver metastases with surgery up-front approach: bi-institutional analysis of 736 consecutive cases. World J Surg. 2012;36(9):2171–2178. doi: 10.1007/s00268-012-1616-y. [DOI] [PubMed] [Google Scholar]
- 11.Vigano L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15(9):2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (N.I.C.E.). Treatment for metastatic colorectal cancer in the liver amenable to treatment with curative intent: Colorectal cancer (update): evidence review D2a. 2020. https://www.nice.org.uk/guidance/ng151/evidence/d2a-treatment-for-metastatic-colorectal-cancer-in-the-liver-amenable-to-treatment-with-curative-intent-pdf-253058083672. [PubMed]
- 13.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2019. [DOI] [PMC free article] [PubMed]
- 14.Integraal Kankercentrum Nederland (I.K.N.L.) Guideline Colorectaal Carcinoom. 2019. https://www.oncoline.nl/colorectaalcarcinoom.
- 15.Viganò L, Pedicini V, Comito T, Carnaghi C, Costa G, Poretti D, et al. Aggressive and multidisciplinary local approach to iterative recurrences of colorectal liver metastases. World J Surg. 2018;42(8):2651–2659. doi: 10.1007/s00268-018-4525-x. [DOI] [PubMed] [Google Scholar]
- 16.Cervantes A, Adam R, Rosello S, Arnold D, Normanno N, Taieb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Petrowsky H, Gonen M, Jarnagin W, Lorenz M, DeMatteo R, Heinrich S, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235(6):863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225(1):51–60. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamoto T, Hashimoto T, Miyata A, Shimada K, Maruyama Y, Makuuchi M. Repeat hepatectomy after major hepatectomy for colorectal liver metastases. J Gastrointest Surg. 2020;24(2):380–387. doi: 10.1007/s11605-019-04154-8. [DOI] [PubMed] [Google Scholar]
- 20.Dijkstra M, Nieuwenhuizen S, Puijk RS, Timmer FEF, Geboers B, Schouten EAC, et al. Thermal ablation compared to partial hepatectomy for recurrent colorectal liver metastases: an Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers. 2021;13(11). [DOI] [PMC free article] [PubMed]
- 21.Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16(9):2385–2390. doi: 10.1245/s10434-009-0492-7. [DOI] [PubMed] [Google Scholar]
- 22.Heise D, Bayings W, Tuinhof A, Eickhoff R, Kroh A, Ulmer F, et al. Long-term outcome and quality of life after initial and repeat resection of colorectal liver metastasis: a retrospective analysis. Int J Surg. 2017;48:281–285. doi: 10.1016/j.ijsu.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Hellingman T, Kuiper BI, Buffart LM, Meijerink MR, Versteeg KS, Swijnenburg RJ, et al. Survival benefit of repeat local treatment in patients suffering from early recurrence of colorectal cancer liver metastases. Clin Colorectal Cancer. 2021;20(4):e263–e272. doi: 10.1016/j.clcc.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Dijkstra M, Nieuwenhuizen S, Puijk RS, Geboers B, Timmer FEF, Schouten EAC, et al. The role of neoadjuvant chemotherapy in repeat local treatment of recurrent colorectal liver metastases: a systematic review and meta-analysis. Cancers. 2021;13(3):378. doi: 10.3390/cancers13030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238(6):871–883. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NVMO. PASKWIL-criteria aangepast. Med Oncol. 2016;12.
- 28.Vigano L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276–1286. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 29.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- 30.IBM Corp. Released 2019. IBM® SPSS® Statistics for Windows, Version 26.0. Armonk: IBM Corp.
- 31.R Core Team. R: a language and environment for statistical computing. R for Windows version 4.0.3. R Foundation for Statistical Computing, Vienna, Austria. 2019.
- 32.Specialisten FM. SONCOS Normeringsrapport—multidisciplinaire normering oncologische zorg in Nederlands. 2022.
- 33.Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg. 2019;269(1):120–126. doi: 10.1097/SLA.0000000000002319. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 35.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13(12):2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence (N.I.C.E.). Radiofrequency ablation for colorectal liver metastases. 2009. https://www.nice.org.uk/guidance/IPG327.
- 37.National Institute for Health and Care Excellence (N.I.C.E.). Microwave ablation for treating liver metastases. 2016. https://www.nice.org.uk/guidance/IPG553.
- 38.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 39.Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone for liver metastasis from colorectal cancer: JCOG0603 study. J Clin Oncol. 2020. 4005.
- 40.Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43(14):2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayez N, Van Der Stok EP, Grünhagen DJ, Rothbarth J, Van Meerten E, Eggermont AM, Verhoef C. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: Clinical risk score as possible discriminator. Eur J Surg Oncol. 2015;41(7):859–867. doi: 10.1016/j.ejso.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Zhu D, Zhong Y, Wei Y, Ye L, Lin Q, Ren L, et al. Effect of neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. PLoS ONE. 2014;9(1). [DOI] [PMC free article] [PubMed]
- 44.Tanaka K, Adam R, Shimada H, Azoulay D, Lévi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90(8):963–969. doi: 10.1002/bjs.4160. [DOI] [PubMed] [Google Scholar]
- 45.Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7(1):109–115. doi: 10.1016/S1091-255X(02)00121-X. [DOI] [PubMed] [Google Scholar]
- 46.Adam R, Wicherts DA, de Haas RJ, Aloia T, Levi F, Paule B, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26(10):1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 47.Vigano L, Galvanin J, Poretti D, Del Fabbro D, Gentile D, Pedicini V, et al. Percutaneous ablation of post-surgical solitary early recurrence of colorectal liver metastases is an effective "test-of-time" approach. Updates Surg. 2021. [DOI] [PubMed]
- 48.Wiering B, Oyen WJ, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, et al. Long-term global quality of life in patients treated for colorectal liver metastases. Br J Surg. 2011;98(4):565–571. doi: 10.1002/bjs.7365. [DOI] [PubMed] [Google Scholar]
- 49.Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 2011;13(11):774–782. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dijkstra M, Nieuwenhuizen S, Puijk RS, Timmer FEF, Geboers B, Schouten EAC, et al. Repeat local treatment of recurrent colorectal liver metastases, the role of neoadjuvant chemotherapy: an Amsterdam Colorectal Liver Met Registry (AmCORE) based study. Cancers (Basel). 2021;13(19). [DOI] [PMC free article] [PubMed]
- 51.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247(1):118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 52.Mehta NN, Ravikumar R, Coldham CA, Buckels JAC, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34(7):782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007;12(7):825–839. doi: 10.1634/theoncologist.12-7-825. [DOI] [PubMed] [Google Scholar]
- 54.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24(31):4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 55.Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17(11):2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 56.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243(1):1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valdimarsson VT, Hellberg K, Brismar TB, Sparrelid E, Sturesson C. Repeat procedures for recurrent colorectal liver metastases: analysis of long-term liver regeneration and outcome. Cancer Manag Res. 2019;11:2617–2622. doi: 10.2147/CMAR.S191653. [DOI] [PMC free article] [PubMed] [Google Scholar]