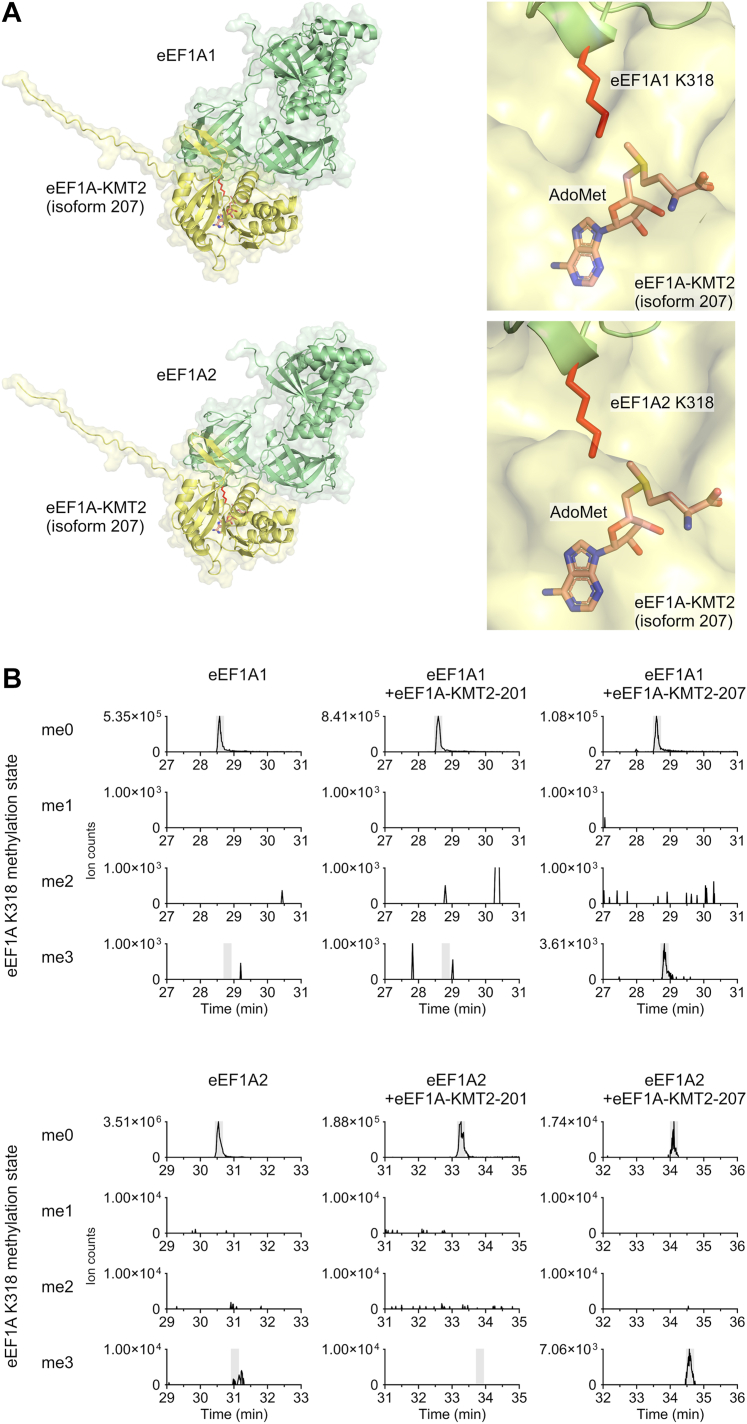
Figure 6.
eEF1A-KMT2 isoform 207 methylates eEF1A1 and eEF1A2 in vitro.A, left: top-ranked AlphaFold-Multimer models of eEF1A-KMT2-207:eEF1A1 and eEF1A-KMT2-207:eEF1A2 complexes shown as cartoon and surface. Right: eEF1A1/2 K318 is bound proximal to the predicted AdoMet-binding site of eEF1A-KMT2-207. AdoMet is shown as sticks, K318 is shown as red sticks, and eEF1A-KMT2-207 is shown as yellow, semi-transparent surface. B, eEF1A-KMT2-207 methylates eEF1A1 and eEF1A2 at K318 in vitro, while eEF1A-KMT2-201 does not. Purified eEF1A1 or eEF1A2 (2.2 μM) were incubated without any enzyme or with eEF1A-KMT2-201 or eEF1A-KMT2-207 (3 μM) in the presence of AdoMet for 18 h at 37 °C. Proteins were separated by SDS-PAGE (see Fig. S20), and eEF1A1 and eEF1A2 gel bands were then digested by AspN and analyzed by LC-MS/MS. Shown are extracted ion chromatograms (XICs) for the triply-charged peptide DNVGFNVKNVSVK (K318 underlined) in its un-, mono-, di-, or tri-methylated states.