Figure 1. Participant Flow Diagram.
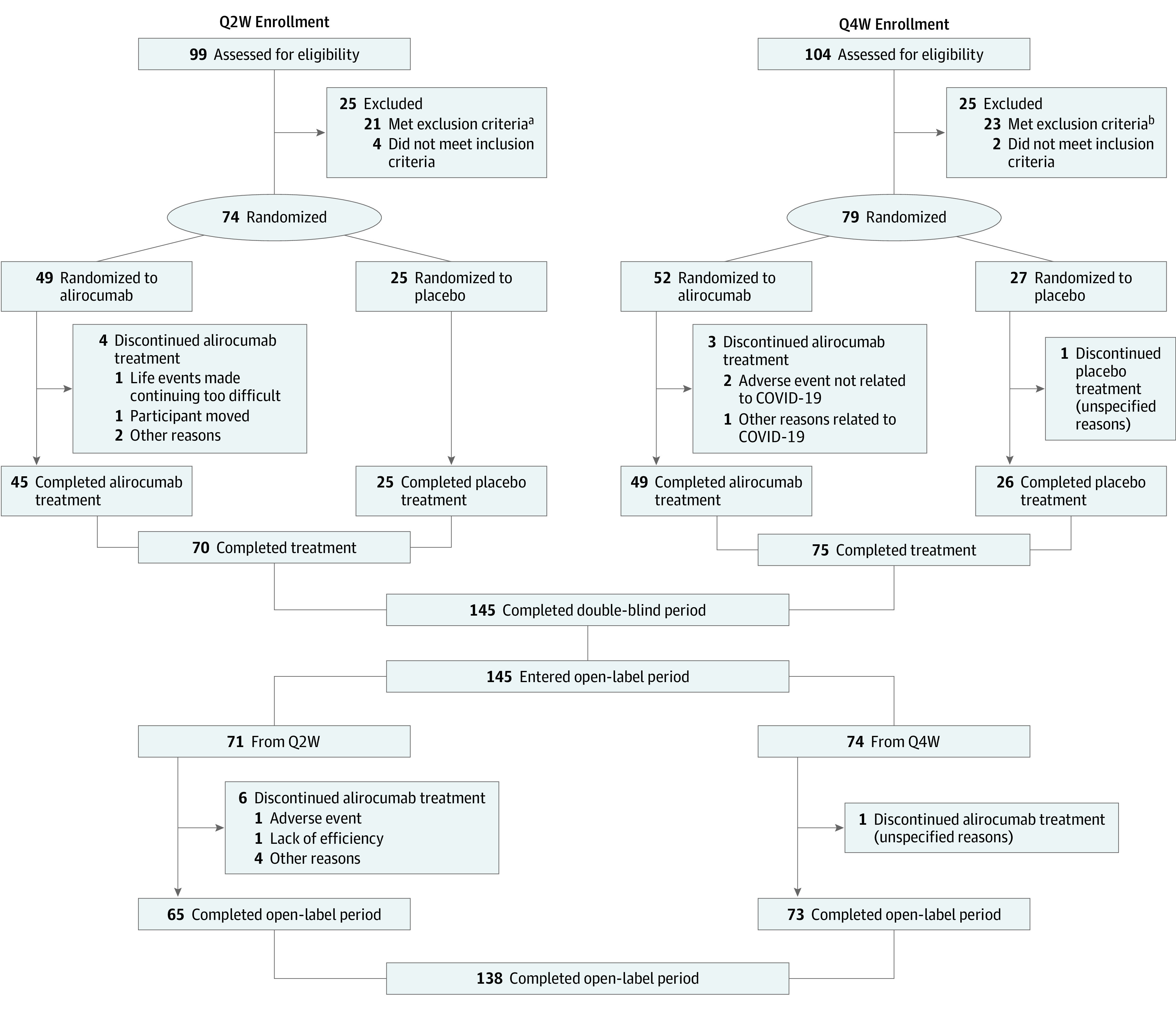
Q2W indicates dosing once every 2 weeks; Q4W, dosing once every 4 weeks.
aIncludes 12 patients (12.1%) with low-density lipoprotein cholesterol less than 130 mg/dL (to convert to mmol/L, multiply by 0.0259) after the patient had been receiving stable lipid-modifying treatment for at least 4 weeks; 5 patients (5.1%) whose parents withdrew consent during the screening period; 3 patients (3.0%) aged 8 to 9 years not at Tanner stage 1 or aged 10 to 17 years not at Tanner stage 2 or higher; 1 patient (1.0%) with uncontrolled type 1 or 2 diabetes (ie, hemoglobin A1C levels above local guidelines or equivalent); and 1 patient (1.0%) with creatine phosphokinase 3 times the upper limit of normal or higher.
bIncludes 14 patients (13.5%) with low-density lipoprotein cholesterol less than 130 mg/dL after the patient had been receiving stable lipid-modifying treatment for at least 4 weeks; 4 patients (3.8%) with any abnormal clinical condition or disease during screening that would hinder study completion or any other condition in the investigator’s opinion as making the patient inappropriate for the study; 3 patients (2.9%) whose parents withdrew consent during the screening period; and 2 patients (1.9%) aged 8 to 9 years not at Tanner stage 1 or aged 10 to 17 years not at Tanner stage 2 or higher.
