Abstract
Objective: Cholinesterase inhibitors (ChEIs) are widely used for the treatment of Alzheimer’s disease (AD); however, their cholinergic side effects on the cardiovascular system are still unclear. In this study, we aimed to examine the side effects caused by donepezil, rivastigmine, and galantamine on cardiac rhythm and postural blood pressure changes in elderly patients with AD. Methods: Of 204 consecutive elderly patients who were newly diagnosed with AD, 162 were enrolled and underwent comprehensive geriatric assessments. The electrocardiographs (ECGs) and blood pressures were recorded at the baseline and 4 weeks after the dose of 10 mg/d of donepezil, 10 cm2/d of rivastigmine, and 24 mg/d of galantamine. Results: There were no changes relative to the baseline in any of the ECG parameters or arterial blood pressure with any of the administered ChEIs. Conclusion: It was demonstrated that none of the 3 ChEIs were associated with increased negative chronotropic, arrhythmogenic, and hypotensive effects for the elderly patients with AD.
Keywords: donepezil, transdermal rivastigmine, galantamine, cardiac safety, Alzheimer disease, elderly patients
Introduction
The cholinesterase inhibitors (ChEIs) including donepezil, rivastigmine, and galantamine are currently considered to be the first-line treatment of Alzheimer’s disease (AD). 1 Although the target organ for these drugs is the brain, the heart is also rich in cholinesterases and their inhibition may adversely affect cardiac function. These cardiac adverse effects, including bradycardia, heart block, and QT prolongation with or without a history of cardiac disease, can emerge as vagotonic effects due to ChEIs. 2,3 QT prolongation may lead to life-threatening ventricular arrhythmias (ie, torsade de pointes and ventricular fibrillation). 4
Donepezil hydrochloride is a reversible, noncompetitive, piperidine-type ChEI, 1 and rivastigmine is also a centrally acting ChEI that stops the metabolization of acetylcholine esterase and butyrylcholine esterase. 5,6 The third one, galantamine, is similar to other centrally acting ChEIs, and it is also associated with allosteric potentiation of the nicotinic receptor N-methyl-d-aspartate and facilitation of synaptic transmission. 7,8 Although all the 3 ChEIs are widely used for the treatment of AD and known to be well tolerated, cholinergic-dependent cardiac side effects of these drugs have been reported. 1 –8
Until now, possible cardiac adverse effects of each ChEI were separately evaluated. 9 –11 However, cardiac effects of these drugs per se have not been analyzed in elderly patients with AD. When the increased prevalence of cardiac disease among the elderly individuals is taken into account, the importance of potential cardiac adverse events related to these drugs is more apparent. Therefore, this study was conducted to compare the cardiac effects of all ChEIs including donepezil, rivastigmine, and galantamine in the elderly patients with AD.
Methods
A total of 204 patients with diagnosed AD were enrolled in this study. Before participation, written informed consent was obtained from each participant and legally authorized representatives according to the local guidelines. The investigation confirms to the Declaration of Helsinki.
Patients were diagnosed with Diagnostic and Statistical Manual of Mental disorder (Fourth Edition, Text Revision; DSM-IV-TR) criteria for primary degenerative dementia of the Alzheimer type and the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association criteria 12 for probable AD. During the study, patients who were treated with cardiostimulatory drugs such as β-adrenoceptor agonists, thyroxine, phosphodiesterase inhibitors, calcium sensitizers, and atrioventricular node blocking drugs, such as, β-blockers, calcium channel blockers, digoxin, and amiodarone were excluded from the study. In addition, the patients with pacemakers were excluded as well. There was no alteration in patients’ medications during the study period.
Exogenous variables that can influence the blood pressure including food intake, exercise, smoking, and the ingestion of caffeine were avoided in 60 minutes before evaluation, and both the blood pressure measurements and electrocardiographs (ECGs) were recorded at the same time. After patients rested quietly for 5 minutes in a quiet and warm setting, their blood pressure measurements were taken in the sitting position with a mercury sphygmomanometer with the proper-sized cuff. 13 All patients were examined with a comprehensive geriatric assessment 9 and a 12-lead surface ECG measurement was recorded with an ECG 1350-K (Nihon Kohden Corporation, Tokyo, Japan) using 25 mm/s paper speed and standardized at 0.1 mV/mm after the patients had rested for at least 10 minutes in a supine position.
The patients with AD were treated with a flexible 4 weekly donepezil tablet (Aricept, Pfizer, Istanbul, Turkey) dosage titration regimen up to 10 mg/d, rivastigmine patch (Exelon patch, Novartis, Istanbul, Turkey) dosage titration regimen up to 10 cm2/d, and galantamine extended release tablet (Reminyl PRC, Janssen-Cilag, Istanbul, Turkey) dosage titration regimen up to 24 mg/d. ECG parameters and blood pressure measurements were recorded at the baseline and 4 weeks after the dose of 10 mg/d donepezil, 10 cm2/d rivastigmine, and 24 mg/d galantamine were given.
ECG parameters, including heart rate, PR interval (PR), QT interval (QT), and corrected QT interval (QTc) and QRS duration were calculated automatically by the apparatus. The QT interval was corrected for the heart rate by using Bazett’s formula 14 and more than 450 milliseconds was defined QTc prolongation. 15
Statistical Analysis
Power and sample size calculation
To detect an increase in QTc interval at α = .05, and power = 0.95, the minimal sample size was required as 20 patients for donepezil, 39 patients for rivastigmine patch, and 31 patients for galantamine (Power and sample size calculator, version 3.0, Vanderbilt University, Nashville, Tennessee).
Statistical analyses were performed using the SPSS software, version 11.5 (SPSS Inc, Chicago, Illinois). Demographics and baseline characteristics were reported as the number (n) and percentage (%) for nominal variables and as the mean ± standard deviation (SD) for continuous variables. The Kolmogorov-Smirnov test was used to determine the distribution characteristics of the variables. Because the differences and its SD among the groups did not show normal distribution, all the comparisons were tested for statistical significance by using the Wilcoxon test. The differences were considered to be significant at P < .05.
Results
A total of 204 patients were enrolled in the study, but only 162 of them completed the study. The number of patients who dropped out was 42; 10 patients had ChEIs intolerance due to refractory nausea, vomiting, and diarrhea; 14 patients due to initiation of treatment with cardiostimulatory drugs; and 18 patients were lost to follow-up. Therefore, we studied 75 male and 87 female elderly individuals with newly diagnosed AD. The demographics and baseline characteristics of the patients are given in Table 1.
Table 1.
Mean Demographics and Other Baseline Characteristics of Patients
| Donepezil (n = 56) | Rivastigmine (n = 52) | Galantamine (n = 54) | |
|---|---|---|---|
| Age, years a | 74.7 ± 6.3 | 77.1 ± 7.2 | 77.8 ± 5.9 |
| Gender, M/F | 26/30 | 24/28 | 25/29 |
| HT, % | 71.4 | 73.0 | 70.3 |
| CAD, % | 39.2 | 34.6 | 31.4 |
| MMSE a | 19.3 ± 5.3 | 18.7 ± 5.9 | 20.1 ± 4.1 |
| CDT a | 1.8 ± 1.2 | 2.2 ± 0.8 | 2.38 ± 0.9 |
| IADLs a | 8.0 ± 5.6 | 8.7 ± 4.6 | 9.5 ± 5.8 |
Abbreviations: HT, hypertension; CAD, coronary artery disease; MMSE, Mini-Mental State Examination (scored of 30); CDT, clock drawing test (scored of 4); IADLs, instrumental activities of daily living (scored of 17); SD, standard deviation.
aMean ± SD.
All comparisons were analyzed excluding the dropouts. The data of dropouts were not considered and thus could not affect the results, even though the mean values of all available data for the dropouts were similar to those who continued.
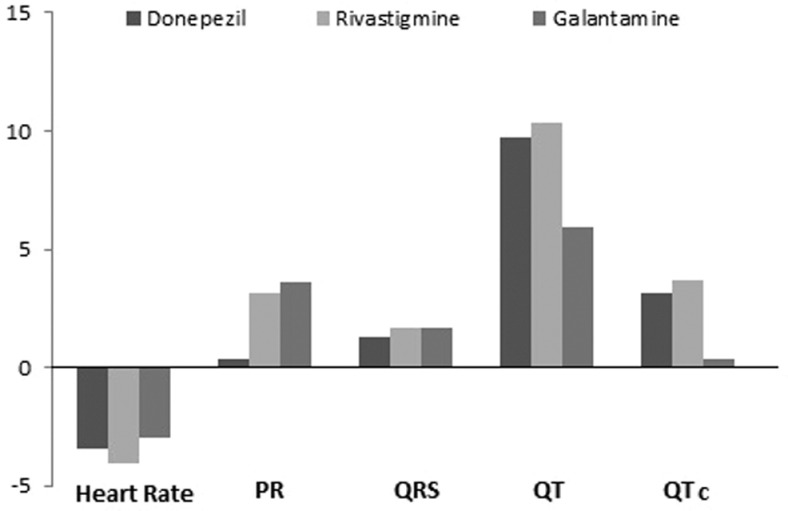
When compared with the baseline, there were no changes, in any of the ECG parameters in all the three treatment groups except HR and QT in the rivastigmine-treated patients (P < .04 and P < .03, respectively; Table 2). On the other hand, when compared with the mean change from baseline for each ChEIs treated, there were no changes in any of the ECG parameters (f = 0.082, P = .92 for HR; f = 0.337, P = .714 for PR; f = 0.009, P = .991 for QRS; f = 0.238, P = .789 for QT; f = 0.162, P = .851 for cQT; Figure 1).
Table 2.
Mean a ECG Parameters and Blood Pressures in Patients Treated With ChEIs
| Baseline | Donepezil 10 mg/d | P value | Baseline | Rivastigmine 10 cm2/day | P value | Baseline | Galantamine 24 mg/d | P value | |
|---|---|---|---|---|---|---|---|---|---|
| HR, beat/min | 77 ± 13 | 73 ± 12 | NS | 74 ± 16 | 70 ± 12 | 0.033 | 72 ± 14 | 69 ± 10 | NS |
| PR, ms | 163 ± 23 | 163 ± 28 | NS | 174 ± 34 | 177 ± 31 | NS | 166 ± 28 | 169 ± 23 | NS |
| QRS, ms | 88 ± 22 | 89 ± 23 | NS | 92 ± 21 | 94 ± 19 | NS | 94 ± 22 | 96 ± 21 | NS |
| QT, ms | 373 ± 46 | 382 ± 34 | NS | 392 ± 44 | 402 ± 39 | 0.023 | 395 ± 41 | 400 ± 36 | NS |
| QTc, ms | 417 ± 28 | 420 ± 29 | NS | 427 ± 31 | 431 ± 38 | NS | 425 ± 30 | 425 ± 27 | NS |
| SBP, mm Hg | 128 ± 15 | 125 ± 17 | NS | 133 ± 17 | 132 ± 18 | NS | 138 ± 17 | 142 ± 23 | NS |
| DBP, mm Hg | 77 ± 7 | 78 ± 9 | NS | 79 ± 10 | 75 ± 9 | NS | 80 ± 8 | 75 ± 10 | NS |
Abbreviations: HR, heart rate; PR, PR interval; QRS, QRS duration; QT, QT interval; QTc, corrected QT interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; ChEI, cholinesterase inhibitor; ECG, electrocardiograms; NS, nonsignificant.
a Mean ± SD, P > .05.
Figure 1.
Mean changes in ECG parameters from baseline in the each treatment group. The mean changes in ECG parameters from baseline for each treatment group were not significant (P >.05 for each comparison). PR indicates PR interval; QRS, QRS duration; QT, QT interval; QTc, corrected QT interval.
The arterial blood pressure of the patients remained unchanged according to the baseline in all the patients (P > .05 for each comparison; Table 2).
Discussion
In this study, it was demonstrated that none of the ChEIs was associated with increased negative chronotropic, arrhythmogenic, or hypotensive effects in elderly patients with AD and was not superior to each other, with regard to vagotonic effects of the ChEIs.
It is known that a prolongation of the QT and QTc intervals is related to electrical instability and risk of ventricular arrhythmogenesis, 16 and age-associated degenerative changes in the conduction system, as in the entire body, a large percentage of older people have complex arrhythmias. 9 Although, the target organ for donepezil, rivastigmine, and galantamine is the brain, the heart is also rich in cholinesterases and their inhibition may adversely affect cardiac function, especially in elderly patients. 2 Therefore, this potentially high-risk elderly patients should be kept in mind when they are treated with ChEIs, which may also have an effect on the cardiac conduction system. In our previous studies, it was reported that galantamine, donepezil, and oral rivastigmine did not affect the ECG parameters. Moreover, arterial blood pressure compared with the baseline in the elderly patients with AD and the usual dosages of galantamine and donepezil seemed to be well tolerated in these elderly patients. 9,10,17 However, until now, it has not been clearly shown whether or not any of galantamine, donepezil, and transdermal rivastigmine is superior to each other, in terms of the vagotonic effects in elderly patients with AD.
In addition to our previous studies, this study demonstrated that the heart rate decreased and the QT interval prolonged while PR interval, QRS duration, and QTc interval remained unchanged in elderly individuals treated with rivastigmine patch. However, it was also demonstrated that when compared with the mean ECG changes from baseline for each treatment group, all the ECG changes in rivastigmine patch-treated patients were similar to donepezil- and galantamine-treated patients.
In the previous studies, it was reported that bradycardia, complete atrioventricular block, and QT prolongation were seen in the patients taking all the 3 ChEIs. 18 –23 Therefore, there has been significant anxiety among prescribers regarding the potential for cardiac adverse effects associated with ChEIs in elderly patients with AD. 24 The present study differs from these reported cases, as it shows that galantamine, donepezil, and transdermal rivastigmine therapies have no significant effect on the ECG parameters including heart rate, PR, QT, and QTc intervals, and QRS duration in elderly patients with AD compared with the baseline and each other. When the age-associated changes in the heart, which increase the risk of arrhythmia, 9 are taken into account, it is obvious how important it is not to have negative chronotropic, arrhythmogenic, and hypotensive effects on the elderly patients with AD. We think that comorbidities and medications may have been responsible for these ChEIs-induced ECG changes encountered in previous studies. 18 –23
In addition, in this study, it has been shown that transdermal rivastigmine, both a butyrylcholinesterase and an acetylcholinesterase inhibitors, unlike donepezil and galantamine, has also similar effects on cardiac conduction and blood pressure in elderly patients with AD as in those treated with others, despite the high density of butyrylcholinesterase in the heart. 25
As it would not be ethical to treat patients with a placebo following the diagnosis of AD, comparisons between the premedication values (the baseline) and medication values of the same patients were made. There was no placebo group. Furthermore, the duration may be a limitation of the study, as these results include only 2 months of maximum dose of these drugs.
In conclusion, it was demonstrated that donepezil, rivastigmine patch, and galantamine have neither negative chronotropic and arrhythmogenic effects nor hypotensive effect on the elderly patients with AD. However, when physicians prescribe these drugs, they should be aware of the comorbidities, especially cardiac conduction disease and medications of the elderly patients.
Acknowledgments
The authors thank Omer Uysal for help in statistical evaluation and Rumeyza Kazancioglu for help in editing the manuscript.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received no financial support for the research, authorship, and/or publication of this article
References
- 1. Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805–2810. [DOI] [PubMed] [Google Scholar]
- 2. Masuda Y. Cardiac effect of cholinesterase inhibitors used in Alzheimer’s disease-from basic research to bedside. Curr Alzheimer Res. 2004;1(4):315–321. [DOI] [PubMed] [Google Scholar]
- 3. Malone DM, Lindesay J. Cholinesterase inhibitors and cardiovascular disease: a survey of old age psychiatrists’ practice. Age Ageing. 2007;36(3):331–333. [DOI] [PubMed] [Google Scholar]
- 4. Keren A, Tzivoni D, Gavish D, Levi J, Gottlieb S, Benhorin J, et al. Etiology, warning signs and therapy of torsade de pointes. A study of 10 patients. Circulation. 1981;64(6):1167–1174. [DOI] [PubMed] [Google Scholar]
- 5. Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible study cholinesterase inhibitor. Clin Ther. 2003;25(6):1631–1653. [DOI] [PubMed] [Google Scholar]
- 6. Farlow M, Lilly LM. Rivastigmine: an open labeled observational study of safety and effectiveness in treating patients with Alzheimer disease for up to 5 years. BMC Geriatr. 2005;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schrattenholz A, Pereira EF, Roth U, Weber KH, Albuquerque EX, Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol.1996;49(1):1–6. [PubMed] [Google Scholar]
- 8. Schilström B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. [DOI] [PubMed] [Google Scholar]
- 9. Isik AT, Bozoglu E, Naharci I, Kilic S. Cardiac safety of galantamine in elderly patients with late onset Alzheimer disease. J Am Ger Pharmacotherapy. 2010;8:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Isik AT, Babacan-Yildiz G, Bozoglu E, Yay A, Aydemir E. Cardiac safety of donepezil in elderly patients with Alzheimer disease. Intern Med. 2012;51:575–578. [DOI] [PubMed] [Google Scholar]
- 11. Morganroth J, Graham S, Hartman R, Anand R. Electrocardiographic effects of rivastigmine. J Clin Pharmacol. 2002;42(5):558–568. [DOI] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of hypertension for clinic ambulatory and self-blood pressure measurement. J Hypertens. 2005;23(4):697–701. [DOI] [PubMed] [Google Scholar]
- 14. Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 15. Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc-interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–367. [DOI] [PubMed] [Google Scholar]
- 16. Elming H, Holm E, Jun L, Torp-Pedersen C, Køber L, Kircshoff M, et al. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998;19(9):1391–1400. [DOI] [PubMed] [Google Scholar]
- 17. Isik AT, Bozoglu E, Naharci MI, Eker D. Cardiac safety of rivastigmine in patients with late onset Alzheimer’s disease. J Geriatr Geriatr Neuropsychiat 2010;1:21–26 [Google Scholar]
- 18. Suleyman T, Tevfik P, Abdulkadir G, Ozlem S. Complete atrioventricular block and ventricular tachyarrhythmia associated with donepezil. Emerg Med J. 2006;23(8):641–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka A, Koga S, Hiramatsu Y. Donepezil-induced adverse side effects of cardiac rhythm: 2 cases report of atrioventricular block and Torsade de Pointes. Intern Med. 2009;48(14):1219–1223. [DOI] [PubMed] [Google Scholar]
- 20. Kayrak M, Yazici M, Ayhan SS, Koc F, Ulgen MS. Complete atrioventricular block associated with rivastigmine therapy. Am J Health Syst Pharm. 2008;65(11):1051–1053. [DOI] [PubMed] [Google Scholar]
- 21. Walsh E, Dourish J. Prolonged QT interval with rivastigmine. Br J Psychiatry. 2002;180:466. [DOI] [PubMed] [Google Scholar]
- 22. Leentjens AF, Kragten JA. Complete atrioventricular block during galantamine therapy. Ned Tijdschr Geneeskund. 2006;150(10):563–566. [PubMed] [Google Scholar]
- 23. Nelson MW, Buchanan RW. Galantamine-induced QTc prolongation. J Clin Psychiatry. 2006;67(1):166–167. [DOI] [PubMed] [Google Scholar]
- 24. Rowland JP, Rigby J, Harper AC, Rowland R. Cardiovascular monitoring with acetylcholinesterase inhibitors: a clinical protocol. Adv Psychiatr Treat. 2007;13:178–184. [Google Scholar]
- 25. Chemnitius JM, Sadowski R, Winkel H, Zech R. Organophosphate inhibition of human heart muscle cholinesterase isoenzymes. Chem Biol Interact. 1999;119-120:183–192. [DOI] [PubMed] [Google Scholar]