ABSTRACT
All life forms have evolved to respond appropriately to various environmental and internal cues. In the animal kingdom, the prototypical regulator class of such cellular responses is the Rel homology domain proteins including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Fungi, the close relatives of animals, have also evolved with their own NF-κB-like regulators called velvet family proteins to govern cellular and chemical development. Here, we conducted a detailed investigation of the taxonomic broad presence of velvet proteins. We observed that velvet proteins are widely distributed in the fungal kingdom. Moreover, we have identified and characterized 21 major velvet clades in fungi. We have further revealed that the highly conserved velvet domain is composed of three distinct motifs and acts as an evolutionarily independent domain, which can be shuffled with various functional domains. Such rearrangements of the velvet domain have resulted in the functional and type diversity of the present velvet regulators. Importantly, our in-deep analyses of the primary and 3D structures of the various velvet domains showed that the fungal velvet domains can be divided into two major clans: the VelB and the VosA clans. The 3D structure comparisons revealed a close similarity of the velvet domain with many other eukaryotic DNA-binding proteins, including those of the Rel, Runt, and signal transducer and activator of transcription families, sharing a common β-sandwich fold. Altogether, this study improves our understanding of velvet regulators in the fungal kingdom.
IMPORTANCE
Fungi are the relatives of animals in Opisthokonta and closely associated with human life by interactive ways such as pathogenicity, food, and secondary metabolites including beneficial ones like penicillin and harmful ones like the carcinogenic aflatoxins. Similar to animals, fungi have also evolved with NF-κB-like velvet family regulators. The velvet proteins constitute a large protein family of fungal transcription factors sharing a common velvet domain and play a key role in coordinating fungal secondary metabolism, developmental and differentiation processes. Our current understanding on velvet regulators is mostly from Ascomycota fungi; however, they remain largely unknown outside Ascomycota. Therefore, this study performed a taxonomic broad investigation of velvet proteins across the fungal kingdom and conducted a detailed analysis on velvet distribution, structure, diversity, and evolution. The results provide a holistic view of velvet regulatory system in the fungal kingdom.
KEYWORDS: velvet regulators, fungi, DNA-binding, Rel homology domain, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), fungal development, secondary metabolism
INTRODUCTION
Life forms have evolved complex adaptations and sensory mechanisms to respond appropriately to various environmental and internal cues (1, 2). For instance, animals have evolved with an elaborate development, inflammation, and immune system for self-defense controlled by various mono- and multi-protein assemblies of the Rel homology domain proteins, including the well-known NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) family (3, 4). The NF-κB family consisting of many members has been implicated in a wide range of cellular processes in animals by forming a variety of homodimers or heterodimers to respond to external stimuli (3, 5, 6). Fungi, the close relatives of animals, as they both belong to Opisthokonta with a common ancestor existing approximately 1 billion years ago, have also evolved with specific velvet family regulators with an NF-κB-like DNA-binding domain (4, 7).
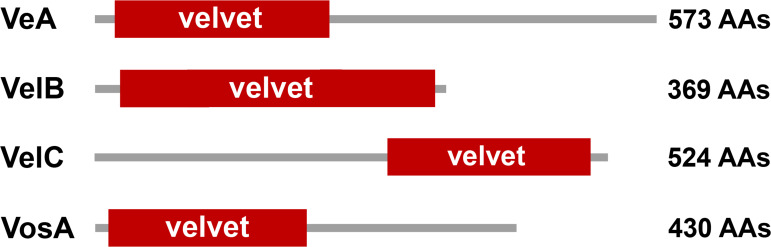
The velvet proteins constitute a large protein family of fungal transcription factors sharing a common velvet domain and play varied roles in coordinating fungal secondary metabolism, developmental and differentiation processes (8–11). In the model organism Aspergillus nidulans, the four well-known members VeA, VelB, VelC, and VosA have been identified and characterized (Fig. 1). The phenotypic outcomes of the four velvet members in A. nidulans were summarized in Table 1. Briefly, the founding member VeA was initially described in the model A. nidulans in the 1960s as a strain harboring the veA1 point mutation producing more conidia and fewer fruiting bodies than the wild-type strain (12). Moreover, veA-deletion mutant failed to produce any sexual fruiting bodies even under favorable dark conditions, while veA overexpression resulted in the constitutive formation of sexual fruiting bodies (13). Much later, the second characterized member VosA was found to be essential for the viability of spores (14). Soon after, the deletion mutant of velB (velvet-like protein B) was reported with a similar phenotype to that of veA (15, 16). A functional study of VelC, the fourth member of the velvet family in A. nidulans, suggested that it functioned as an activator of sexual development (17).
Fig 1.
The domain architectures of the four velvet proteins in A. nidulans. AAs, amino acid residues.
TABLE 1.
Summary for the phenotypic outcomes of the velvet mutants in A. nidulansa
| Velvet member | Asexual development | Sexual development | Secondary metabolism | References | |
|---|---|---|---|---|---|
| veA | KO | Increased conidia production | No sexual structures | Decreased penicillin production; lack of sterigmatocystin (ST) production; increased brownish pigment accumulation | (13, 18, 19) |
| OE | Decreased conidia production | Increased sexual structure formation | Decreased penicillin production | ||
| velB | KO | Reduced conidia production; increased conidial germination rates; defect of conidia viability; lack of trehalose in conidia | No sexual fruit bodies | Reduced and delayed ST production; increased brownish pigment accumulation | (15, 16, 20) |
| OE | A twofold increase of asexual spore production | No excessive production of cleistothecia | |||
| velC | KO | Increased conidia production | Reduced production of sexual fruiting bodies (cleistothecia) | (17) | |
| OE | Equivalent amounts of asexual spores | Increased formation of cleistothecia | |||
| vosA | KO | Defect of conidia viability; lack of trehalose in conidia; sensitive to various stresses; increased β-glucan accumulation in conidia; uncontrolled activation of asexual development | Defective sexual fruiting bodies; decreased ascospore viability; lack of trehalose biogenesis; decreased tolerance of ascospores to thermal and oxidative stresses | A slight increase of ST production in ascospores | (14, 21) |
| OE | Inhibition of sporulation | ||||
The deletion and overexpression phenotypes of mutants were compared to the wild type. KO, knockout mutants; OE, overexpression mutants.
Additionally, a series of studies involving the characterization of velvet homologs also revealed the importance of the velvet regulatory system in various biological processes in a wide range of fungal species. The functional study of FvVE1 in Fusarium verticillioides was the first characterization of a veA homolog gene in a fungal species outside the genus Aspergillus (22). Later, lots of related studies were performed in other Ascomycetes, such as Acremonium chrysogenum (23), Cochliobolus heterostrophus (24), Histoplasma capsulatum (25), Mycosphaerella graminicola (26), Neurospora crassa (27, 28), and Penicillium chrysogenum (29). In Basidiomycetes, three velvet homologs umv1, umv2, and umv3 have been functionally characterized in Ustilago maydis (30). Besides, in silico analysis of fungal genomes showed that velvet proteins are present across several different fungal taxa (31, 32).
Crystal structure analysis of the VosA velvet domain revealed a structural similarity with the Rel homology domain of the mammalian transcription factor NF-κB (4). In addition, similar to NF-κB members, velvet proteins can form a variety of homodimers, heterodimers, or complexes with various partners having distinct roles in fungal biology. For instance, the heterotrimeric complex VelB-VeA-LaeA governs sexual development and secondary metabolism in A. nidulans (15). The VelB homodimer functions as a positive regulator of asexual development, whereas the VosA homodimer plays a negative regulatory role in conidiation during vegetative growth and the early phase of conidiophore formation in A. nidulans (16). The VosA-VelB complex is a master regulatory unit for structure, metabolism, and physiology in both asexual and sexual spores in A. nidulans (21, 33). Currently, numerous velvet complexes in the genus Aspergillus have been reported and summarized (34). Outside the genus Aspergillus, the velvet homodimers, heterodimers, or complexes have also been identified in a wide range of fungi, such as Botrytis cinerea (35), Neurospora crassa (28), Penicillium chrysogenum (29, 36), and Verticillium dahliae (37).
In this study, we performed a taxonomically broad survey of velvet proteins in the fungal kingdom to reveal their distribution, protein size, domain architecture, etc. Then, we classified the velvet proteins into different clades based on their phylogenetic relationship, and compared the conserved motifs, and 3D structures among the different velvet clades. Results suggested that velvet proteins are blooming in the fungal kingdom but also beyond the kingdom. The velvet domain is highly conserved with three characteristic motifs and could combine with different functional domains to form various velvet proteins. We further revealed that the fungal velvet domains could be divided into two clans (VelB clan and VosA clan). At last, we propose that the velvet domain together with the DNA-binding domains of the Rel, Runt, and signal transducer and activator of transcription (STAT) families sharing a similar β-sandwich fold should belong to the same DNA-binding domain superfamily. Altogether, this study presents a holistic view on the diversity, structure, and evolution of velvet proteins.
RESULTS
Diversification of velvet proteins in the fungal kingdom
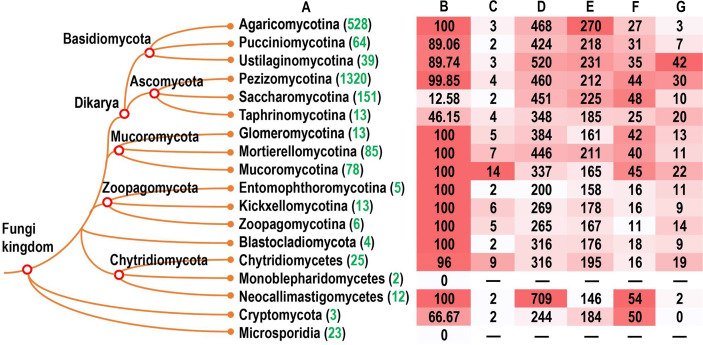
To address the diversity of velvet proteins in the fungal kingdom, their homologs were investigated in a wide range of fungi covering the phyla Ascomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Cryptomycota, Microsporidia, Mucoromycota, and Zoopagomycota. The distribution information of velvet genes in terms of their frequency and family diversity in genomes was summarized in the tested fungi (Fig. 2).
Fig 2.
Distribution features of the velvet family in the fungal kingdom. (A) The genome numbers of each fungal group accessed in MycoCosm (38) are highlighted in green. (B) The percentage of genomes having velvet genes. (C) The mode of velvet gene numbers per genome in each fungal group. The detailed information is provided in Fig. 3. (D) The average length of velvet proteins (amino acid residues) in each fungal group. The detailed information is provided in Fig. 4. (E) The average length of velvet domains (amino acid residues) in each fungal group. (F) The percentage of N-terminal side located velvet domains in each fungal group. The detailed information is provided in Fig. 5. (G) The percentage of C-terminal side located velvet domains in each fungal group.
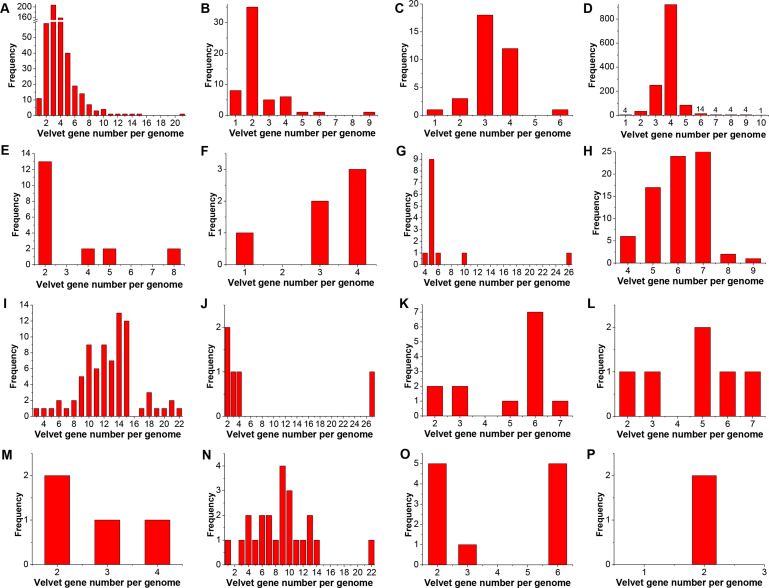
In general, velvet proteins are widespread in the tested fungal phyla from higher fungi to lower fungi, except for their absence in Microsporidia (Fig. 2A and B). Among the tested taxonomic groups, most genomes contained velvet genes, but it was observed that no velvet genes were detected in the current two Monoblepharidomycete genomes, and only part of Ascomycota yeasts contained velvet genes. The mode of velvet gene number per genome varied among the taxonomic groups with the count ranging from 2 to 14 (Fig. 2C). Furthermore, the frequency distribution of velvet gene number per genome was also compared among different fungal taxonomic groups (Fig. 3). In general, the distribution varies greatly by the taxonomic groups. For instance, among the 1,320 Pezizomycotina genomes, approximately 70% contained four velvet genes and 19% contained three velvet genes, whereas approximately 39% of the 528 Agaricomycotina genomes harbored three velvet genes and 30% contained four velvet genes. In particular, the quantity of velvet genes outbreaks in the Mucoromycotina genomes and approximately 82% harbored more than 10 velvet genes.
Fig 3.
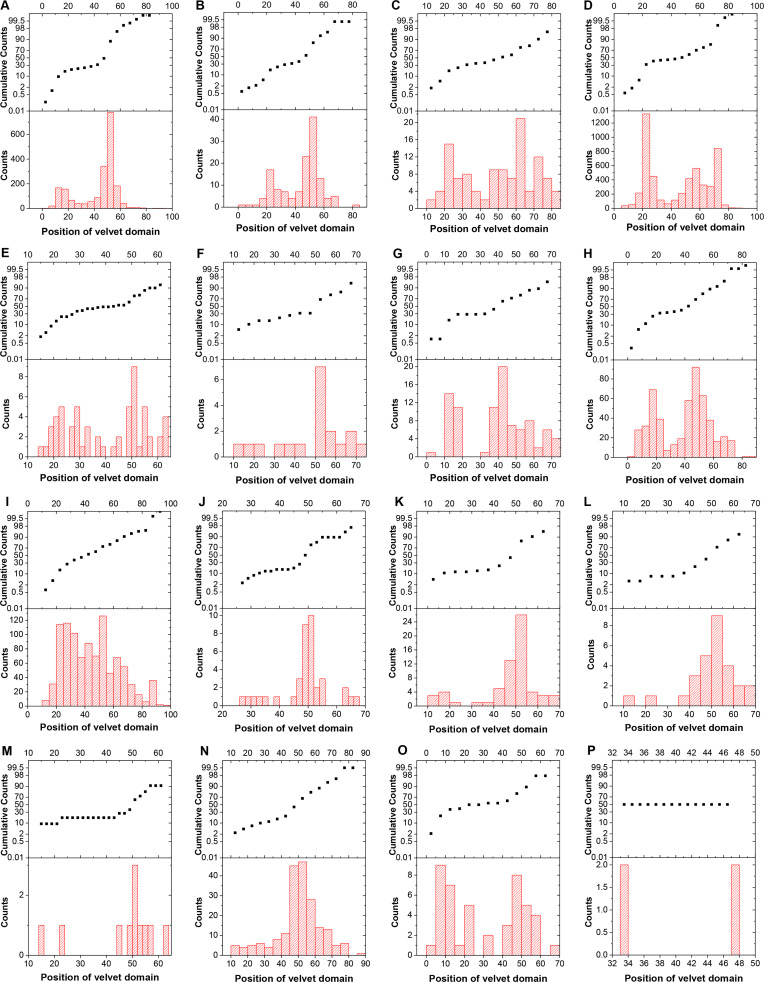
The frequency distribution of velvet gene number per genome in different fungal groups. The genomes without velvet genes were not counted. A, B, and C, respectively, correspond to the groups Agaricomycotina, Pucciniomycotina, and Ustilaginomycotina in the phylum Basidiomycota. D, E, and F, respectively, correspond to the groups Pezizomycotina, Saccharomycotina, and Taphrinomycotina in the phylum Ascomycota. G, H, and I, respectively, correspond to the groups Glomeromycotina, Mortierellomycotina, and Mucoromycotina in the phylum Mucoromycota. J, K, and L, respectively, correspond to the groups Entomophthoromycotina, Kickxellomycotina, and Zoopagomycotina in the phylum Zoopagomycota. M corresponds to the phylum Blastocladiomycota. N and O, respectively, correspond to the groups Chytridiomycetes and Neocallimastigomycetes in the phylum Chytridiomycota. P corresponds to the phylum Cryptomycota.
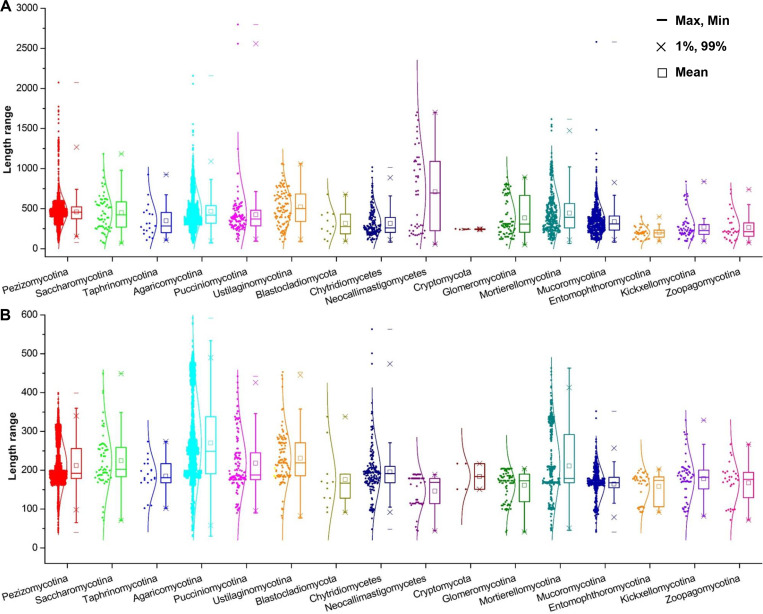
The length distribution of velvet proteins varied significantly both within and among the taxonomic groups, ranging from several hundreds to thousands (Fig. 2D and 4A). The Neocallimastigomycetes velvet proteins showed the longest average length of 709 amino acid residues (AAs); however, a general trend was observed that the average length in higher fungi is longer than that in lower fungi. The velvet domain length is around 200 AAs (Fig. 2E and 4B). The Agaricomycotina velvet proteins harbored velvet domains with the longest average length of 270 AAs. In general, the average length of the velvet domain in higher fungi is longer than that in lower fungi.
Fig 4.
Length box charts of velvet proteins and domains in different fungal groups. The length was calculated as the number of amino acid residues. Normal distribution was used to fit the length distribution. A and B, respectively, correspond to the charts of velvet proteins and domains.
The position of velvet domains was investigated and compared among the taxonomic groups (Fig. 2F, G and 5). As revealed, the velvet domain could be located in the N-terminal side, middle, or C-terminal side of proteins, but its position distribution varies among different fungal taxonomic groups. In Ustilaginomycotina and Pezizomycotina, the velvet domains located in the C-terminal side occupy a large proportion with the percentage over 30%; however, on the whole, the percentage of velvet domains in the N-terminal side is higher than that in the C-terminal side in the phyla Ascomycota, Basidiomycota, and Mucoromycota. In the phyla Blastocladiomycota and Zoopagomycota, the velvet proteins are shorter and the velvet domains occupy a large part of the proteins.
Fig 5.
The distribution of velvet domain position in different fungal groups. The position of velvet domain in a protein was calculated as the midpoint of velvet domain divided by the protein length (the number of amino acid residues). The position of velvet domain locating before 40% was defined as N-terminal side of the protein, while that locating after 60% was defined as C-terminal side of the protein and others are middle part of the protein. A, B, and C, respectively, correspond to the groups Agaricomycotina, Pucciniomycotina, and Ustilaginomycotina in the phylum Basidiomycota. D, E, and F, respectively, correspond to the groups Pezizomycotina, Saccharomycotina, and Taphrinomycotina in the phylum Ascomycota. G, H, and I, respectively, correspond to the groups Glomeromycotina, Mortierellomycotina, and Mucoromycotina in the phylum Mucoromycota. J, K, and L, respectively, correspond to the groups Entomophthoromycotina, Kickxellomycotina, and Zoopagomycotina in the phylum Zoopagomycota. M corresponds to the phylum Blastocladiomycota. N and O, respectively, correspond to the groups Chytridiomycetes and Neocallimastigomycetes in the phylum Chytridiomycota. P corresponds to the phylum Cryptomycota.
Grouping of Ascomycota velvet proteins and their features
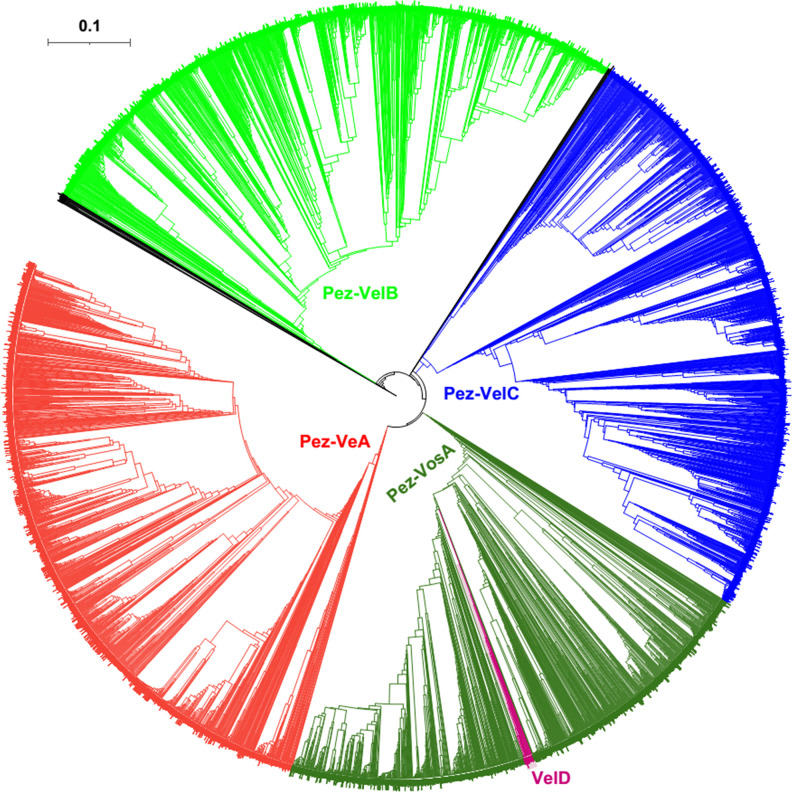
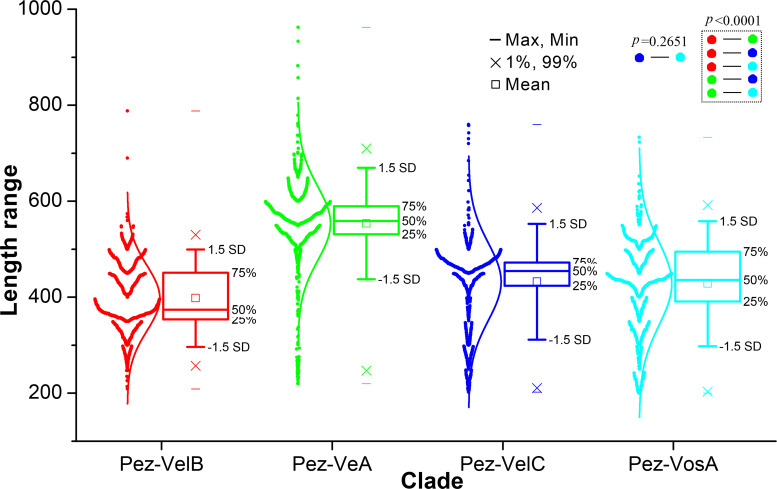
Pezizomycotina constitutes the majority (approximately 90%) of Ascomycota fungi (39). The phylogeny of the Pezizomycotina velvet proteins was analyzed and they were clearly classified into four main clades, Pez-VeA, Pez-VelB, Pez-VelC, and Pez-VosA, based on their phylogenetic relationships (Fig. 6). VelDs, as the fifth velvet member found in most species of Aspergillus section Flavi (40), are gathered in a branch inside the Pez-VosA clade based on the phylogenetic relationship. The length distribution of velvet proteins among the four clades was compared (Fig. 7). It showed that the average protein lengths were 554 AAs in the Pez-VeA clade, 398 AAs in the Pez-VelB clade, 432 AAs in the Pez-VelC clade, and 428 AAs in the Pez-VosA clade. Based on the statistical analysis, except for the comparison between the Pez-VelC clade and the Pez-VosA clade, other two-group comparisons of length distribution indicated an extremely significant departure (P < 0.0001).
Fig 6.
Phylogenetic relationship of the Pezizomycotina velvet proteins. The branch length of the tree is indicated by the scale bar in the upper left corner. The clades of these velvet proteins are indicated by their colors. The figure in high definition is provided as Fig. S1.
Fig 7.
Length distribution of clades Pez-VeA, Pez-VelB, Pez-VelC, and Pez-VosA in Pezizomycotina shown as box plots. The clades were based on the phylogenetic relationship shown in Fig. 6. The length was calculated as the number of amino acid residues of velvet proteins. The normal distribution was used to fit the length distribution. Two-group comparisons were performed using the t-test.
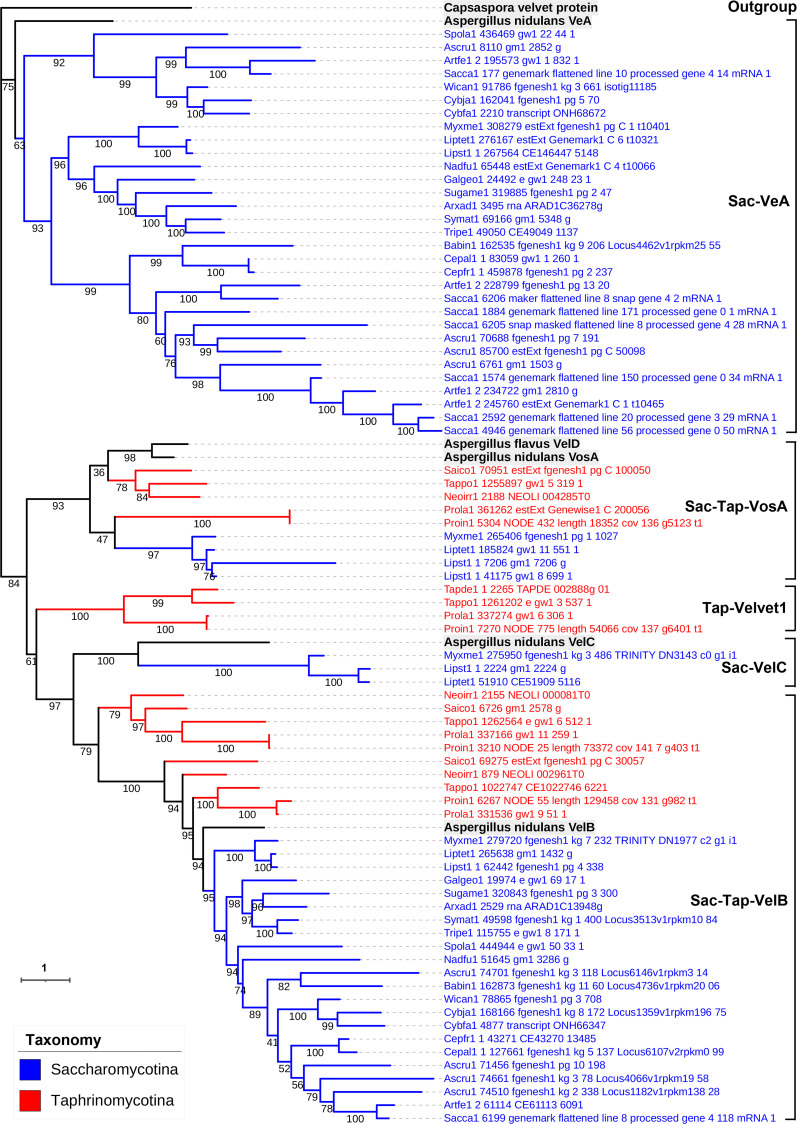
Unlike in Pezizomycotina, velvet genes were detected only in a part of the genomes in Saccharomycotina and Taphrinomycotina (Fig. 2B). The phylogenetic tree of velvet proteins of Saccharomycotina and Taphrinomycotina was constructed (Fig. 8). In general, although Saccharomycotina and Taphrinomycotina are relatives of Pezizomycotina within the phylum Ascomycota, they showed a significant difference in the distribution of velvet clades. As shown in Fig. 8, the Saccharomycotina velvet proteins could also be classified into the clades Sac-VeA, Sac-Tap-VelB, Sac-VelC, and Sac-Tap-VosA, but the clades Sac-VeA and Sac-Tap-VelB are dominant. The Taphrinomycotina velvet proteins were classified into the clades Sac-Tap-VelB, Sac-Tap-VosA, and a new clade named Tap-Velevt1. Unexpectedly, no Taphrinomycotina velvet proteins were detected in the clades VeA and VelC.
Fig 8.
Phylogenetic relationship of Saccharomycotina and Taphrinomycotina velvet proteins. The Capsaspora velvet protein was used as an outgroup. Aspergillus flavus VelD and A. nidulans VeA, VelB, VelC, and VosA were used as references. Bootstrap values for each node are presented. The branch length of each tree is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower left corner. The clades are marked on the right.
Grouping of Basidiomycota velvet proteins and their features
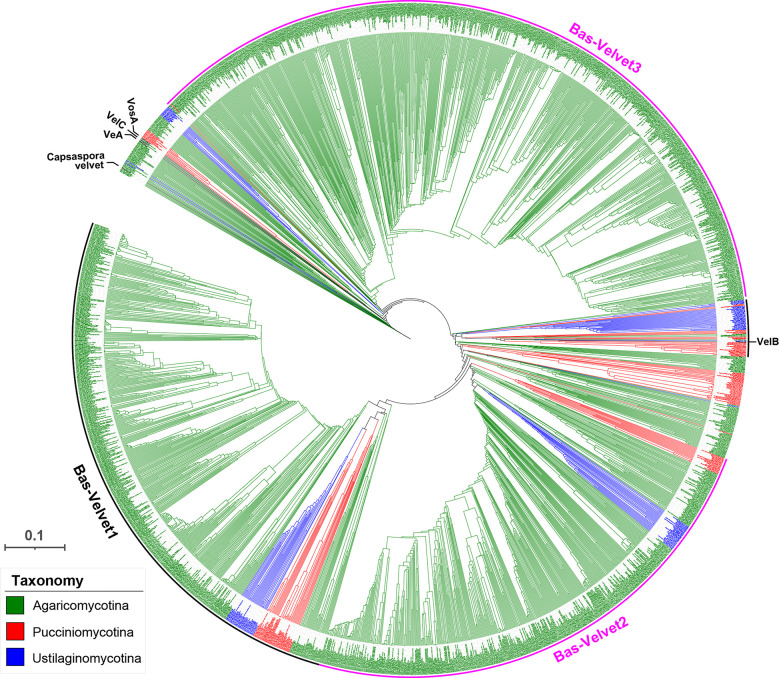
A phylogenetic tree of the Basidiomycota velvet proteins was constructed (Fig. 9). Basidiomycota together with Ascomycota constitutes the subkingdom Dikarya; however, they show extremely different repertoires of velvet members. Based on the phylogenetic relationship, except for a small VelB clade found among the Basidiomycota velvet proteins, no VeA, VelC, and VosA clades formed in the tree. In contrast, three new major clades (Bas-Velvet1, Bas-Velvet2, and Bas-Velvet3) were assigned for the Basidiomycota velvet proteins. The clades Bas-Velvet1 and Bas-Velvet2 consisted of members from the three subphyla Agaricomycotina, Pucciniomycotina, and Ustilaginomycotina. However, the vast majority of members of the clade Bas-Velvet3 are from the subphylum Agaricomycotina. In the VelB clade, the members are primarily from the subphyla Pucciniomycotina and Ustilaginomycotina.
Fig 9.
Phylogenetic relationship of the Basidiomycota velvet proteins. The position of the references Capsaspora velvet protein, A. nidulans VeA, VelB, VelC, and VosA is indicated on the outer. The tree branch length is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower left corner. The clades are marked on the outer. The figure in high definition is provided as Fig. S2 to S7.
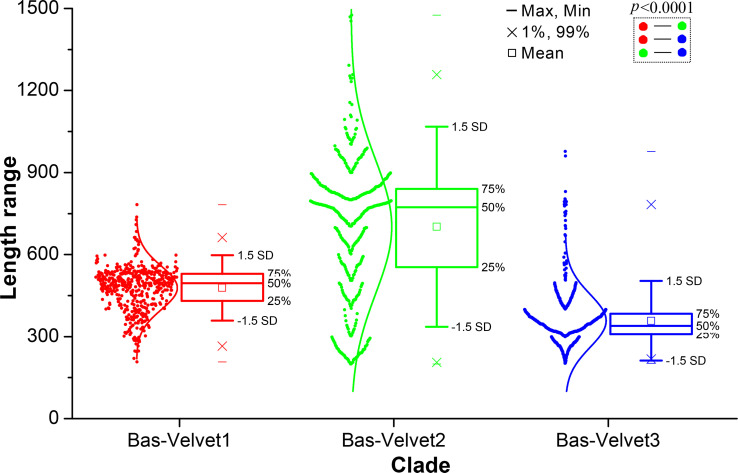
The length distribution of velvet proteins in the three major clades was investigated (Fig. 10). The average protein lengths were 478 AAs in the Bas-Velvet1 clade, 702 AAs in the Bas-Velvet2 clade, and 358 AAs in the Bas-Velvet3 clade. The two-group comparisons of length distribution among the clades indicated an extremely significant departure (P < 0.0001) in the statistical analysis.
Fig 10.
Length distribution of clades Bas-Velvet1, Bas-Velvet2, and Bas-Velvet3 in Basidiomycota shown as box plots. The clades were based on the phylogenetic relationship shown in Fig. 9. The length was calculated as the number of amino acid residues of velvet proteins. The normal distribution was used to fit the length distribution. Two-group comparisons were performed using the t-test.
Grouping of Mucoromycota velvet proteins and their features
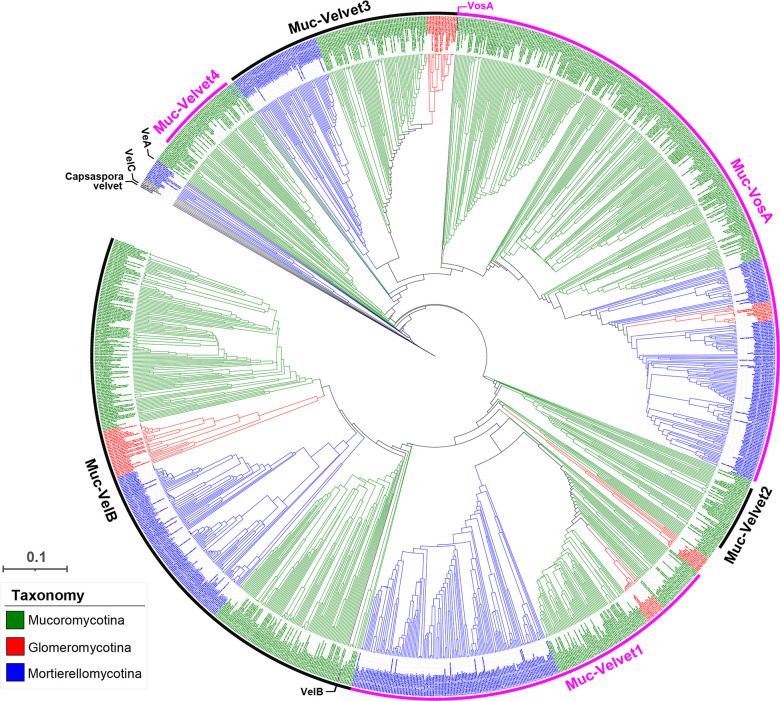
A phylogenetic tree of the Mucoromycota velvet proteins was constructed (Fig. 11). As shown, six major clades (Muc-Velvet1, Muc-Velvet2, Muc-Velvet3, Muc-Velvet4, Muc-VelB, and Muc-VosA) were formed in the tree. The clades Muc-VelB and Muc-VosA are the two large divisions of the Mucoromycota velvet proteins, covering the subphyla Glomeromycotina, Mortierellomycotina, and Mucoromycotina. However, no VeA and VelC clades were formed in the tree. Muc-Velvet1 and Muc-Velvet3 are the two newly allocated clades for the Mucoromycota velvet proteins, and both cover the three subphyla. Muc-Velvet2 and Muc-Velvet4 are the two Mucoromycotina-specific clades.
Fig 11.
Phylogenetic relationship of the Mucoromycota velvet proteins. The Capsaspora velvet protein was used as the outgroup. A. nidulans VeA, VelB, VelC, and VosA were used as references. The branch length of each tree is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower left corner. The clades are marked on the outer. The figure in high definition is provided as Fig. S3.
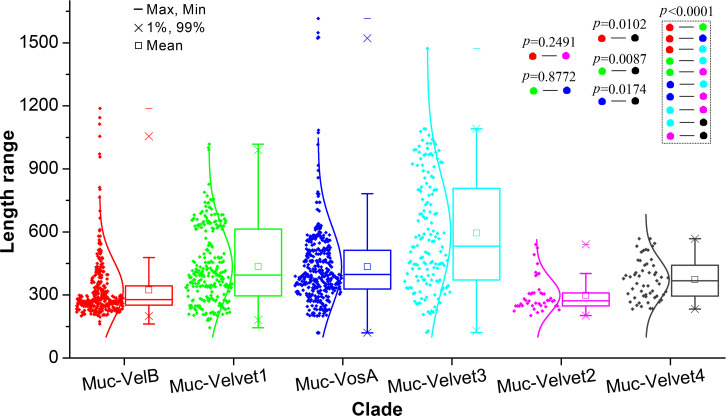
The length distribution of velvet proteins in the six major clades was compared (Fig. 12). The average protein lengths were 436 AAs in the Muc-Velvet1 clade, 299 AAs in the Muc-Velvet2 clade, 595 AAs in the Muc-Velvet3 clade, 373 AAs in the Muc-Velvet4 clade, 323 AAs in the Muc-VelB clade, and 435AAs in the Muc-VosA clade. Based on the two-group comparisons, the length distributions of the Muc-VelB clade vs the Muc-Velvet2 clade and the Muc-Velvet1 clade vs the Muc-VosA clade showed no statistically significant difference, whereas other two-group comparisons revealed statistically significant differences.
Fig 12.
Length distribution of the clades Muc-Velvet1, Muc-Velvet2, Muc-Velvet3, Muc-Velvet4, Muc-VelB, and Muc-VosA in Mucoromycota shown as box plots. The clades were based on the phylogenetic relationship shown in Fig. 11. The length was calculated as the number of amino acid residues of velvet proteins. The normal distribution was used to fit the length distribution. Two-group comparisons were performed using the t-test.
Grouping of Blastocladiomycota, Chytridiomycota, Cryptomycota, and Zoopagomycota velvet proteins and their features
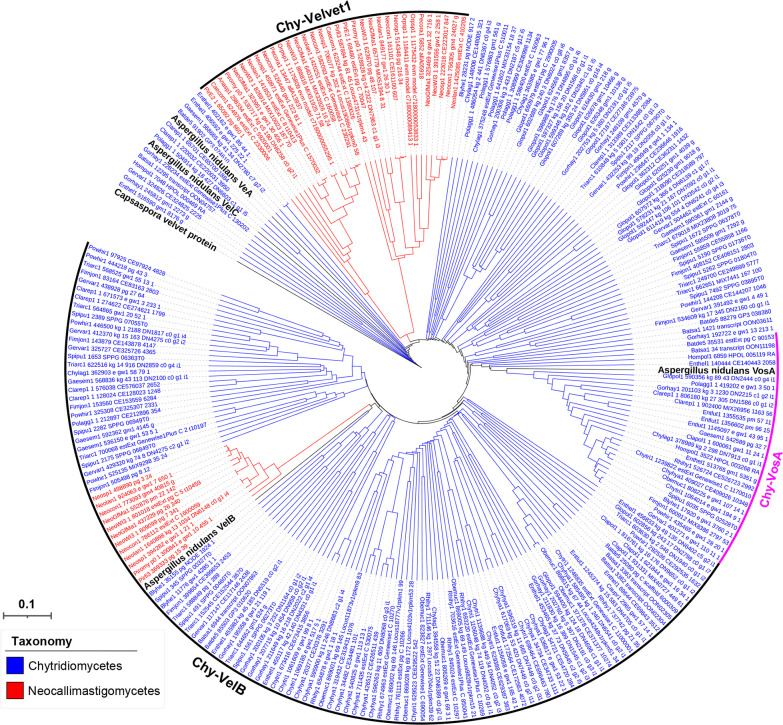
A phylogenetic tree of the Chytridiomycota velvet proteins was constructed (Fig. 13). Based on the phylogenetic relationship, three major clades (Chy-VelB, Chy-VosA, and Chy-Velvet1) were formed among the Chytridiomycota velvet proteins. Chy-VelB is a large clade containing proteins from the classes Chytridiomycetes and Neocallimastigomycetes. The Chy-VosA clade is Chytridiomycetes-specific, whereas the Chy-Velvet1 clade is Neocallimastigomycetes-specific. No VeA and VelC clades were formed in the tree.
Fig 13.
Phylogenetic relationship of Chytridiomycota velvet proteins. The Capsaspora velvet protein was used as the outgroup. A. nidulans VeA, VelB, VelC, and VosA were used as references. They are highlighted in bold. The branch length of each tree is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower left corner. The clades are marked on the right.
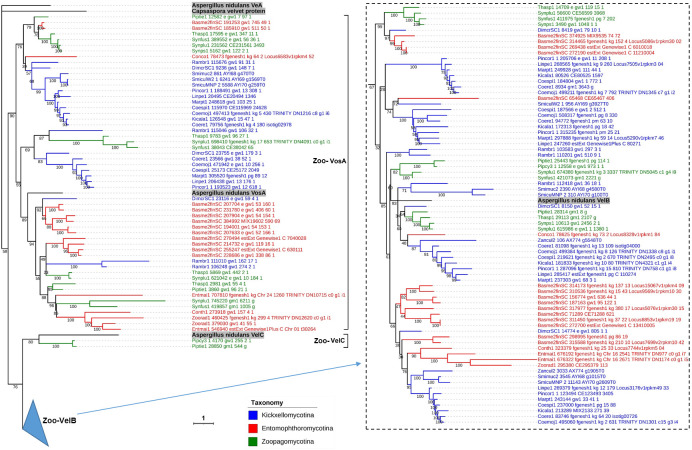
The Zoopagomycota velvet proteins could be grouped into three clades (Zoo-VelB, Zoo-VosA, and Zoo-VelC) (Fig. 14). The clades Zoo-VelB and Zoo-VosA are two large divisions of the Zoopagomycota velvet proteins, covering the subphyla Entomophthoromycotina, Kickxellomycotina, and Zoopagomycotina. The clade Zoo-VelC is a small branch and Zoopagomycotina-specific.
Fig 14.
Phylogenetic relationship of Zoopagomycota velvet proteins. The Capsaspora velvet protein was used as the outgroup. A. nidulans VeA, VelB, VelC, and VosA were used as references. They are highlighted in bold. Bootstrap values for each node are presented. The branch length of each tree is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower middle. The clades are marked on the right of protein IDs. The VelB clade is collapsed in the tree, and expands on the right.
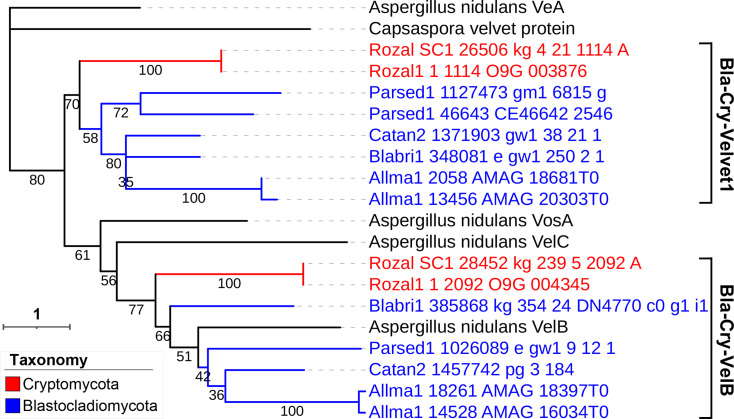
The Blastocladiomycota and Cryptomycota velvet proteins were presented on the same tree (Fig. 15). The velvet proteins were grouped into two clades (Bla-Cry-Velvet1 and Bla-Cry-VelB). Bla-Cry-Velvet1 is a new clade and no VeA, VelC, and VosA clades were found in the tree.
Fig 15.
Phylogenetic relationship of Blastocladiomycota and Cryptomycota velvet proteins. The Capsaspora velvet protein was used as the outgroup. A. nidulans VeA, VelB, VelC, and VosA were used as references. Bootstrap values for each node are presented. The branch length of each tree is indicated by the scale bar in the lower left corner. The taxonomic groups of these velvet proteins are indicated by their colors paraphrasing in the lower middle. The clades are marked on the right of protein IDs.
Comparison of velvet domain features of the 21 clades
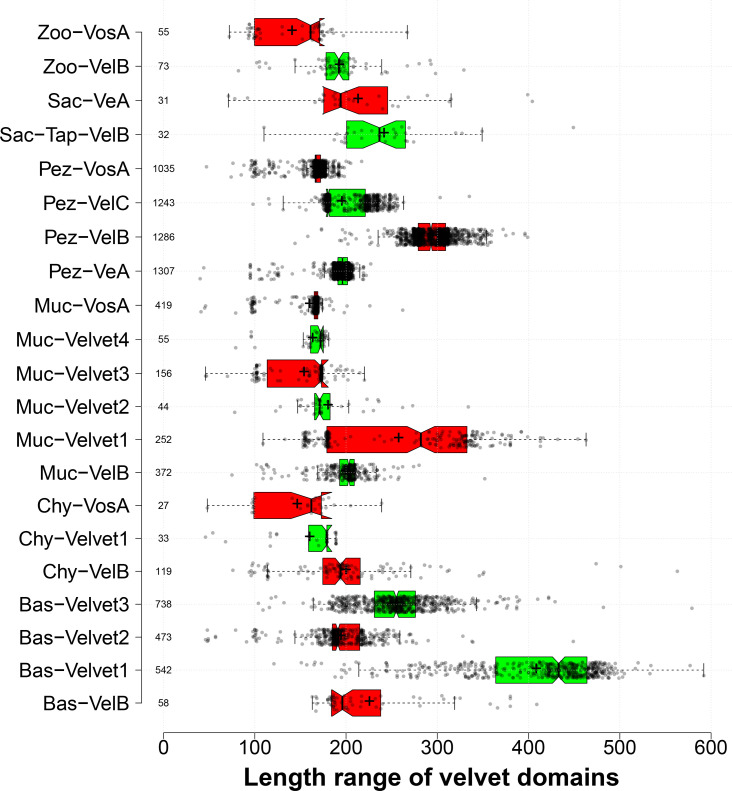
The length distribution of velvet domains from the 21 major clades was compared (Fig. 16). In general, most velvet domains are around 200 AAs, but there are also some individual differences. For example, among the four well-known members in Pezizomycotina, the average length of Pez-VeA velvet domains was 194 AAs, extremely close to that of Pez-VelC velvet domains of 195 AAs, whereas the average length of Pez-VosA velvet domains was much shorter at around 166 AAs, but that of Pez-VelB velvet domains was much longer at around 294 AAs. In particular, the Bas-Velvet1 clade possessed a very long velvet domain with an average length of 408 AAs.
Fig 16.
Length distribution of velvet domains from the 21 major clades shown as box plots. The clades were based on the aforementioned phylogenetic analysis. The length was calculated as the number of amino acid residues of velvet domains. The figure was generated by BoxPlotR (41). Data points are shown in a jittered mode with the Tukey whisker extent. The notches were added to the boxes in the presence of medians, and the symbol + indicates the mean value.
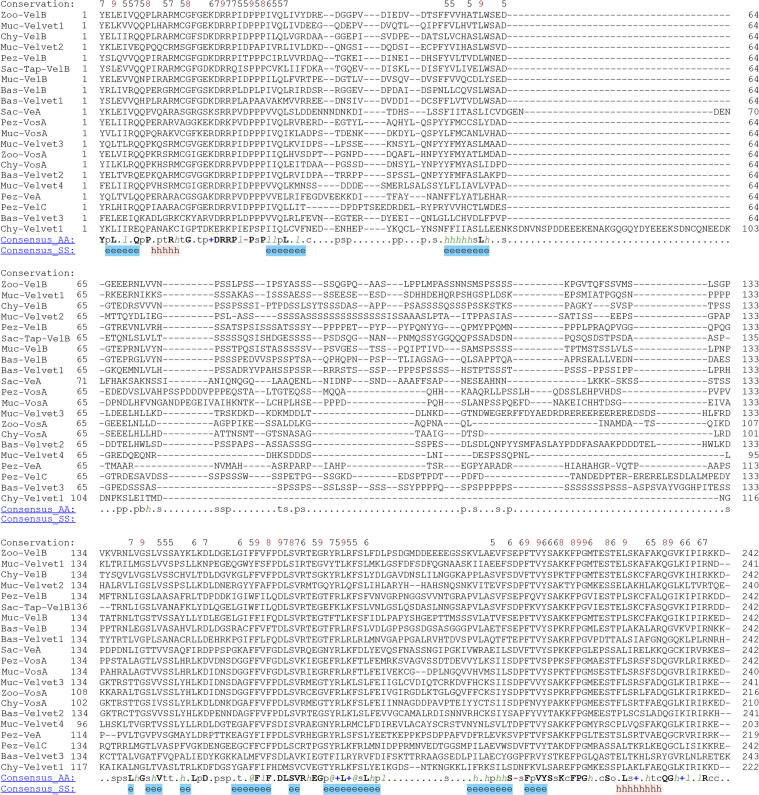
Then, the conserved residues of velvet domains among the 21 major clades were compared (Fig. S4) and the three characteristic motifs were revealed (Fig. 17). In general, the N-terminal region harbors a conserved motif of around 33 residues (termed motif 1) and the C-terminal region contains a characteristic motif of around 36 residues (termed motif 3). The characteristic motif 2 with around 44 residues is close motif 3. The large region between motif 1 and 2 is not conserved in terms of both sequence and length.
Fig 17.
Comparison of the three characteristic motifs of velvet domains among the 21 major clades. The alignment of velvet domains was performed against the profile hidden Markov model of velvet domain PF11754 with 243 residues (https://www.ebi.ac.uk/interpro/entry/pfam/PF11754/) and then subjected to WebLogo (https://weblogo.threeplusone.com/) to generate sequence logos. In the logo, the total stack height represents the information content of residues at that position. The relative height of each residue in the stack is proportional to its frequency at the position, and the residues were sorted so that the most common one was on the top of the stack. The full sequence logos of velvet domains are provided in Fig. S4. The residues are colored according to their chemical properties, of which polar ones G, S, T, Y, and C are in green; neutral ones Q and N are in purple; basic ones K, R, and H are in blue; acidic ones D and E are in red; and hydrophobic ones A, V, L, I, P, W, F, and M are in black. The black balls at the bottom indicate the consensus dominant residues in the 21 clades, and the red balls indicate the other conserved residues revealed by the ConSurf analysis.
The three characteristic motifs embody both commonalities and differences across the 21 velvet domains. On the one hand, the 21 velvet domains share up to 48 conserved sites in their characteristic motifs, 14 of which are with consensus dominant residues across the 21 velvet domains. On the other hand, different velvet members may also be distinguishable by their specific motifs or residues. For example, among the four members of Pezizomycotina, their signatures from the position 140 to 149 are quite different.
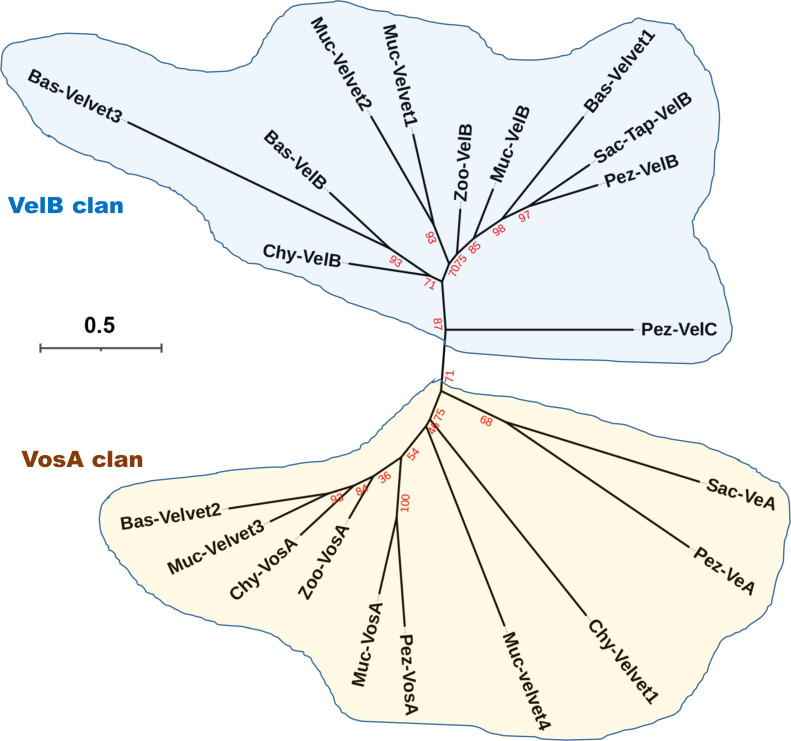
The phylogenetic relationship of the 21 velvet domains was analyzed based on their consensus sequences (Fig. 18). In phylogeny, there are two major clans (VelB and VosA clans) for the fungal velvet domains. Prediction of the subcellular localization of the 21 velvet domains with their consensus sequences using WoLF PSORT suggested their presence in the nucleus or dual localization shuttling between the cytosol and nucleus, and nuclear export signal (NES) motifs were detected in 12 of 21 velvet domain consensus sequences by NESmapper (Fig. S4). However, no nuclear localization signal (NLS) motif was detected in the 21 consensus sequences by NLStradamus.
Fig 18.
Phylogenetic relationship of the 21 velvet domains based on their consensus sequences. Bootstrap values for each node are highlighted in red.
3D structure modeling and comparison of the 21 velvet domains
The 3D structures of the 21 velvet domains were modeled using AlphaFold 2 with their consensus sequences (Fig. S5). The multiple sequence alignment depth and diversity of the 21 consensus sequences generated by ColabFold (Fig. S6) also suggested that the three characteristic motifs (Fig. 17) are much conserved. The predicted lDDT-Cα score per residue of the 21 consensus sequences was used as a measure of their AlphaFold 2 confidence (Fig. S7). In general, the scores of the conserved N- and C-terminal regions are higher than those of the unconserved middle regions.
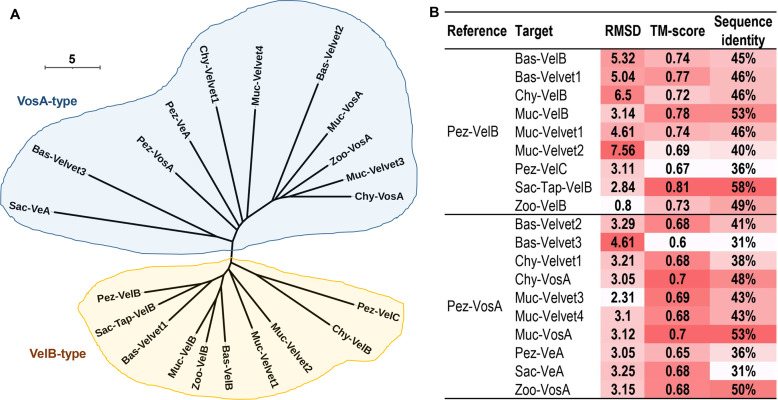
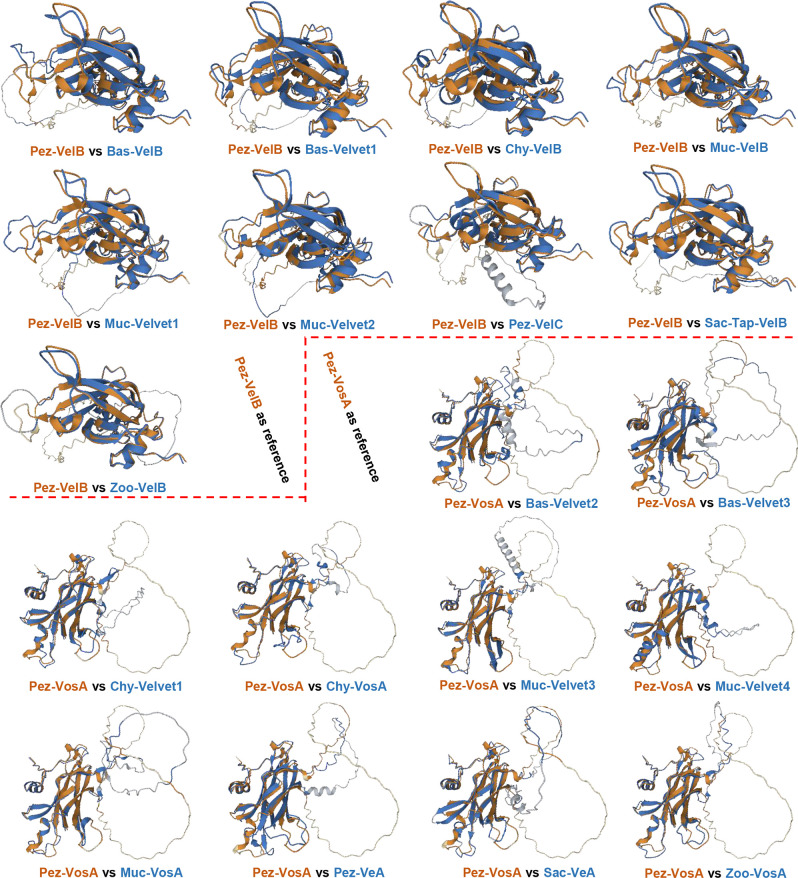
The secondary structures of the 21 velvet domains were aligned (Fig. 19). As the results revealed, the three characteristic motifs of velvet domains are conserved not only in their primary sequences but also in their secondary structures. Then, the global structural similarity of the 21 velvet domains was compared and based on the dendrogram (Fig. 20), the 3D structures of the 21 velvet domains could be divided into two types viz., VelB-type and VosA-type. For further determining the topological similarity among the structures within the VelB-type or VosA-type, the pairwise structure alignment was performed with Pez-VelB and Pez-VosA as references (Fig. 20B and 21). As revealed, in general, the N- and C-terminal regions of the velvet domains share a highly similar protein fold, but the middle regions harbor different loops (Fig. 21). Regarding the TM-scores (template modeling scores) between the reference and target structures (Fig. 20B), all scores are greater than the threshold 0.5, which generally indicates that the proteins have the same fold (42). Meanwhile, a certain positive linear correlation was observed between the TM-score and sequence identity (r = 0.7793).
Fig 19.
Alignment of the 21 velvet domains based on their secondary structures. The predicted 3D structures of the 21 velvet domains (Fig. S5) modeled by AlphaFold 2 with their consensus sequences were submitted to PROMALS3D for structure alignment. Consensus secondary structure (SS) symbols: alpha-helix: h; beta-strand: e. Consensus AA symbols: conserved amino acids are in bold and uppercase letters; aliphatic (I, V, L): l; aromatic (Y, H, W, F): @; hydrophobic (W, F, Y, M, L, I, V, A, C, T, H): h; alcohol (S, T): o; polar residues (D, E, H, K, N, Q, R, S, T): p; tiny (A, G, C, S): t; small (A, G, C, S, V, N, D, T, P): s; bulky residues (E, F, I, K, L, M, Q, R, W, Y): b; positively charged (K, R, H): +; negatively charged (D, E): -; charged (D, E, K, R, H): c.
Fig 20.
3D structure comparison of the 21 velvet domains. (A) The structural similarity dendrogram of the 21 velvet domains. The 3D structures of the 21 velvet domains were submitted to the Dali server with all against all structure comparison for generating their structural similarity dendrogram. (B) The pairwise structure alignment summary of velvet domains with Pez-VelB and Pez-VosA as references. The detailed comparison was given in Fig. 21. For measuring the alignments, the lower the root mean square deviation (RMSD), the better the structure alignment between the pair of structures. TM-score ranges between 0 and 1, and scores >0.5 generally indicate that the proteins have the same fold (42).
Fig 21.
The pairwise structure alignment of velvet domains with Pez-VelB and Pez-VosA as references. The 3D structures of velvet domains were submitted to the Protein Data Bank (PDB) server (https://www.rcsb.org/alignment) for pairwise structure alignment with the jFATCT (rigid) method. The comparison was summarized in Fig. 20B.
Other functional domains of velvet proteins
It is noted that many velvet proteins are much longer than their velvet domains and may also include other functional domains. Therefore, besides velvet domains, other functional domains of velvet proteins were investigated among the 21 clades (Table 2; Table S1). In general, the distribution of functional domains differs by clade. For example, among the four well-known members in Pezizomycotina, 58 types of functional domains were detected in approximately 58.8% of proteins in the Pez-VeA clade, and approximately 40 types of functional domains were detected in >30% of proteins in the clades Pez-VelC and Pez-VosA. However, probably due to the shorter length, only 11.9% of proteins in the Pez-VelB clade detected functional domains. PHA03247 (large tegument protein UL36) was the most frequent domain found in many clades. In the Muc-Velvet1 clade, 16.7% of velvet proteins contained the domain Glyco_transf_49 (glycosyl-transferase for dystroglycan).
TABLE 2.
Summary of the detected functional domains among the 21 major cladesa
| Clade (protein count) | Percentage with detected domains | Type total of detected domains | Main domains and percentages |
|---|---|---|---|
| Bas-VelB (58) | 15.5% | 6 | PHA03247 (3.4%), PHA03307 (3.4%), PLN02217 (3.4%) |
| Bas-Velvet1 (542) | 34.7% | 24 | PHA03247 (15.7%), dnaA (4.2%), Herpes_BLLF1 (2.2%) |
| Bas-Velvet2 (473) | 37.2% | 36 | PHA03247 (14.6%), PRK07764 (5.5%), PRK10263 (2.1%) |
| Bas-Velvet3 (738) | 2.7% | 11 | PHA03247 (0.7%) |
| Chy-VelB (119) | 5.9% | 5 | PHA03247 (2.5%) |
| Chy-Velvet1 (33) | 6.1% | 2 | PRK12678 (3.0%), PTZ00121(3.0%) |
| Chy-VosA (27) | 33.3% | 7 | PHA03247 (11.1%) |
| Muc-VelB (372) | 7.3% | 15 | AdoMet_MTases (1.6%), Smc (1.3%) |
| Muc-Velvet1 (252) | 24.6% | 15 | Glyco_transf_49 (16.7%) |
| Muc-Velvet2 (44) | 4.5% | 1 | PTZ00173 (4.5%) |
| Muc-Velvet3 (156) | 26.3% | 12 | PHA03247 (11.5%), PHA03307 (4.5%) |
| Muc-Velvet4 (55) | 0 | 0 | |
| Muc-VosA (419) | 15.3% | 17 | dnaA (2.9%), PHA03307 (1.9%) |
| Pez-VeA (1307) | 58.8% | 58 | PHA03247 (26.7%), PRK10263 (11.8%), dnaA (2.8%) |
| Pez-VelB (1286) | 11.9% | 28 | PHA03247 (2.3%), PABP-1234 (1.7%), PRK10263 (1.7%) |
| Pez-VelC (1243) | 35.2% | 37 | PHA03247 (15.8%), PRK10263 (6.8%), dnaA (4.4%) |
| Pez-VosA (1035) | 31.4% | 42 | PHA03247 (7.0%), PTZ00395 (4.4%), PABP-1234 (4.1%) |
| Sac-Tap-VelB (32) | 6.3% | 2 | DUF5695 (3.1%), PRK14971 (3.1%) |
| Sac-VeA (31) | 6.5% | 1 | PRK10263 (6.5%) |
| Zoo-VelB (73) | 0 | 0 | |
| Zoo-VosA (55) | 0 | 0 |
Distribution of the velvet proteins outside the fungal kingdom
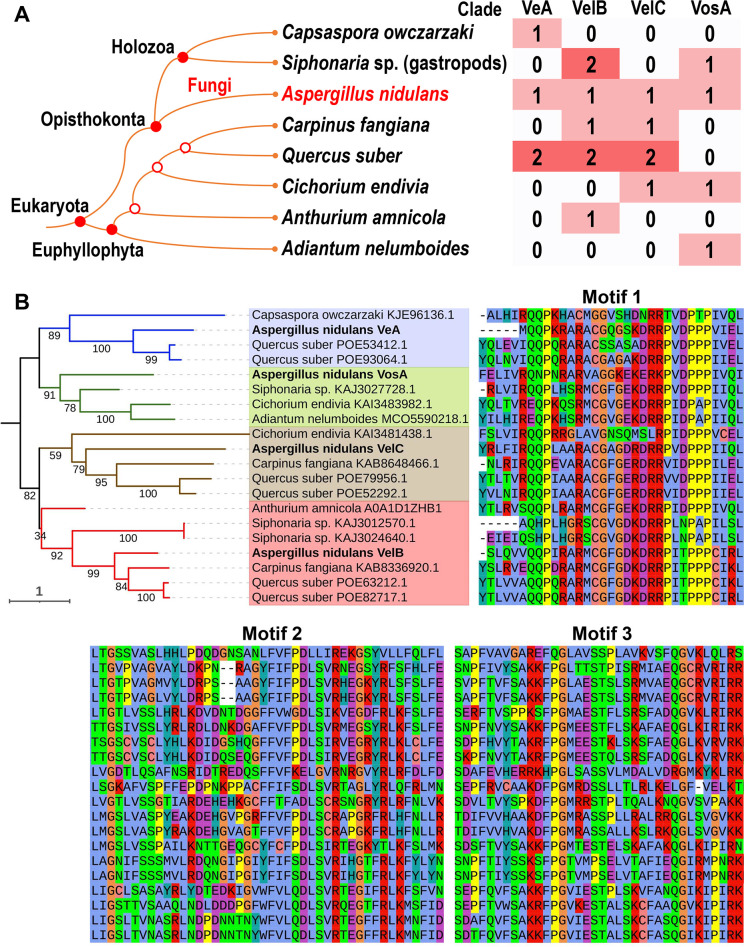
The homologs of velvet proteins outside the fungal kingdom were queried on the protein databases of NCBI (https://www.ncbi.nlm.nih.gov/) and UniProt (https://www.uniprot.org/). Results showed that complete velvet domains were also detected outside the fungal kingdom and the proteins were clearly grouped into the clades VeA, VelB, VelC, and VosA (Fig. 22). In Holozoa, a relative branch of fungi under Opisthokonta, two species Capsaspora owczarzaki (44) and Siphonaria sp. harbor velvet proteins. In Euphyllophyta, velvet proteins were detected in several plant species. Especially in Quercus suber, six velvet proteins classified into three clades were detected. Beyond Eukaryota, several homologs of velvet domains have been detected in Archaea and Bacteria, but their velvet domains are not complete and not considered further.
Fig 22.
Features of velvet proteins outside the fungal kingdom. (A) A cladogram of species beyond the fungi harboring velvet proteins and their distribution. The taxonomic relationship was based on the NCBI taxonomy database (45), and A. nidulans was used as a representative fungus. (B) Phylogenetic relationship of velvet proteins outside the fungal kingdom and their three velvet characteristic motifs. A. nidulans VeA, VelB, VelC, and VosA were used as references and highlighted in bold on the tree. The velvet proteins in the same clade are highlighted with the same background color. The residues are colored with the Clustal X default coloring scheme.
DISCUSSION
A possible evolutionary scenario for the velvet family was reconstructed
Velvet proteins were once considered specific for the fungal kingdom (8, 33, 34). According to our results (Fig. 2), velvet proteins are widely distributed in the fungal kingdom. Furthermore, beyond the fungal kingdom, velvet proteins reach as far as Euphyllophyta. However, at present, it was still difficult to infer the earliest occurrence node of velvet proteins. It was not quite sure whether velvet members of Euphyllophyta are indigenous genes or originated via horizontal gene transfer or contaminated by fungal DNA. Based on the BlastP analysis in the NCBI database, the Euphyllophyta velvet proteins exhibit the closest similarity to their Ascomycete counterparts.
In the fungal kingdom, 21 major clades were classified in this study. Along the evolutionary course of the fungal kingdom, the velvet family underwent gene loss, duplication, and divergence, resulting in its diversification. For instance, most Saccharomycotina genomes showed no velvet genes (Fig. 2), but the Saccharomycotina ancestor should have contained velvet genes and subsequently lost them. Most Mucoromycotina genomes contained more than 10 velvet genes (Fig. 3), and based on their phylogenetic relationship (Fig. 11), the multiple velvet genes probably originated via gene duplications. In general, velvet types vary in different fungal taxa. Based on the phylogenetic analysis, VelB and VosA clades may be very ancient in the fungal kingdom due to their wide presence. The VeA clade appears Ascomycota-specific. The VelD branch of some Aspergillus spp. belongs to the Pez-VosA clade, suggesting that VelD is a variant of Pez-VosA. VelD may have originated from a duplication of vosA in the ancestor of Aspergillus section Flavi, and subsequently diverged with VosA. The VelC clade is flourishing in Ascomycota, but it also presented outside Ascomycota, suggesting its possible earlier presence than Ascomycota.
Velvet proteins are generally constituted by a velvet domain and optional other regions. Based on our results (Table 2), the additional regions of velvet proteins (excluding the velvet domain region) could harbor various functional domains. In other words, the velvet domain could be in the N-terminal side, middle, or C-terminal side and combine with various functional domains to form various velvet proteins, resulting in their diversification in the fungal kingdom. This evolutionary plasticity could serve the specific biological requirements of different fungi adapted to their respective habitats.
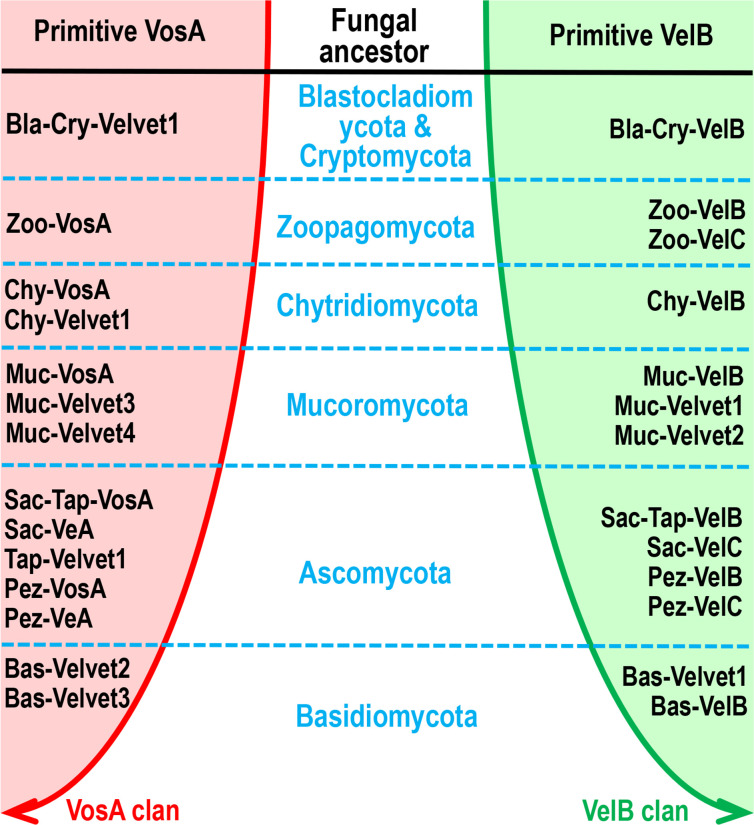
In view of the fact that the conserved velvet domain is shared by the entire velvet family, the velvet domains could be used for tracking their long-term evolution. Therefore, the phylogenetic relationship among fungal velvet clades was analyzed based on their velvet domain primary sequences (Fig. 18) and 3D structures (Fig. 20A). The results of both analyses indicated that the current fungal velvet domains are clearly classified into two clans (named VelB and VosA clans). The list of VelB and VosA clans across the fungal kingdom is summarized in Fig. 23. It shows that all the tested phyla contain members of the VelB and VosA clans. To summarize, primitive VelB and VosA may have existed in the fungal ancestor. Along with the expansion of the fungal kingdom, these two clans were expanding out various velvet clades (Fig. 23).
Fig 23.
Two evolutionary clans of velvet clades in the fungal kingdom. The phylogenetic relationship of fungal velvet clades was based on their velvet domains.
The velvet domain is constituted by three characteristic motifs
It is widely recognized that velvet proteins are diverse but share a highly conserved velvet domain (8). The Pfam database (https://www.ebi.ac.uk/interpro/entry/pfam/PF11754/) provides a velvet domain model with 243 characterized residues. Based on the statistics of velvet domain lengths among different clades (Fig. 16), most velvet domains are of approximate 200 AAs, but some can be even of more than 500 AAs. In this study, all the tested velvet proteins carried only one single velvet domain.
The primary residues of velvet domains were compared among the 21 major fungal velvet clades (Fig. 17 and Fig. S4). Obviously, the three conserved characteristic motifs were found in the velvet domains across different clades. The secondary and 3D structures of the 21 velvet domains were also compared (Fig. 19 to 21). As the results revealed, the three characteristic motifs of velvet domains are conserved not only in their primary sequences but also in their secondary and 3D structures. In other words, these three characteristic motifs constitute the basic skeleton of velvet domains and are probably related to their general functions.
For example, in vivo and in vitro analyses of the A. nidulans VosA velvet domain revealed that the motif 1 region is involved in DNA-binding and several positively charged residues (Lys, Arg) are susceptible to DNA-binding activity (4). As shown, the positively charged residues (Lys, Arg) frequently occupy the 13th, 20th, 22nd, and 23rd positions of motif 1 across different velvet domains (Fig. 17). Currently, much remains to be elucidated regarding the relationship between velvet structure and function, and an analysis of conserved residues or motifs could direct the functional analysis of velvet domains.
The velvet domain exhibits a structural similarity to many DNA-binding proteins
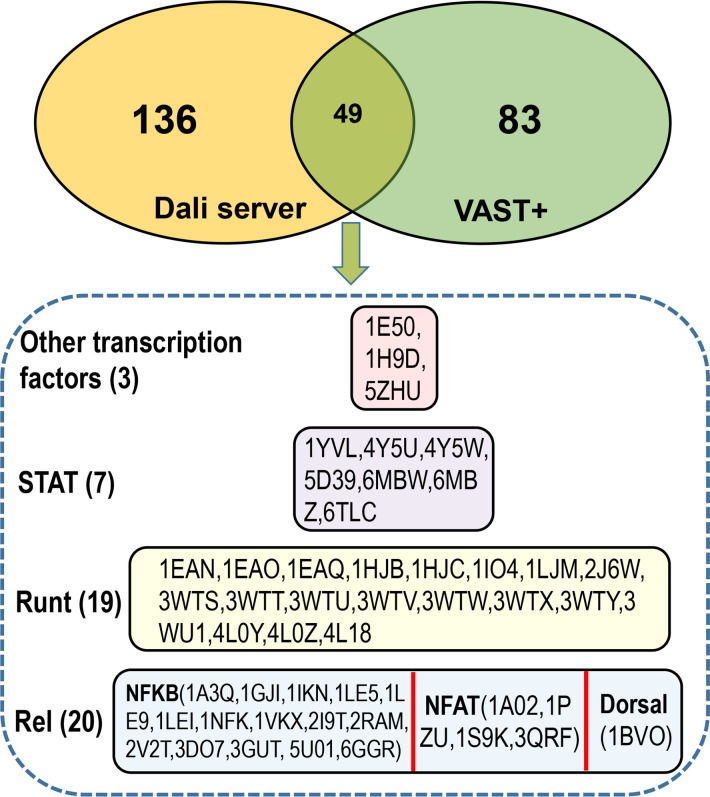
To date, the crystal structures of the VosA homodimer and VosA-VelB complex from A. nidulans have been characterized and the comparison revealed an unexpected structural similarity of the velvet domain with the Rel homology domain of the mammalian transcription factor NF-κB (4). Therefore, we searched the available proteins with 3D structures to examine the significant hits with structural similarities to the velvet domain. In the database of Protein Data Bank (PDB) (https://www.rcsb.org/), hundreds of structurally similar proteins were found using the Dali server by querying the VosA velvet domain (PDB ID: 4N6Q chain A) (Table S2). In the NCBI protein structure database (https://www.ncbi.nlm.nih.gov/Structure/), 132 similar structures of the VosA velvet domain were found using the Vector Alignment Search Tool Plus (VAST+) (Table S3).
The lists of structurally similar proteins were compared between the Dali server and the VAST+ analysis (Fig. 24). The 49 shared proteins were primarily classified into three types of DNA-binding domains (Fig. 24). Consistent with a previous report (4), 20 Rel homology domains from a wide variety of eukaryotic transcription factors such as NF-κB, dorsal, and nuclear factor of activated T-cells (46, 47) were found to have similar 3D structures with the VosA velvet domain. Other remarkable hits included 19 Runx1 Runt domains and 7 DNA-binding domains of the STAT proteins.
Fig 24.
Comparison of structurally similar proteins of the VosA velvet domain by the Dali server and VAST+. The detailed lists of structurally similar proteins by the Dali server and the VAST+ analysis are provided in Table S2 and S3. The upper part is a Venn diagram between the Dali server analysis and the VAST+ analysis. The lower part is a detailed list of the 49 shared proteins by the Dali server and the VAST+ analysis. The PDB IDs are listed inside the box.
Similar to velvet proteins, these three types of transcription factors are also large families with various members that can form different dimers and play diverse roles in the regulation of cellular functions (3, 48, 49). In the database of InterPro (50), the DNA-binding domains of the Rel, Runt, and STAT families belong to the same β-sandwich type superfamily (https://www.ebi.ac.uk/interpro/entry/InterPro/IPR008967/). Therefore, the velvet domain with a common β-sandwich fold (4) should also belong to this DNA-binding domain superfamily. However, they may be not phylogenetically related because of their low amino acid sequence similarities with sequence identities ranging from 9% to 22 %. Probably, the shared 3D structure similarity among the velvet domain and other DNA-binding domains originated from the directed structure convergent evolution in their long-term independent interaction with DNA.
In summary, we conducted a taxonomically broad survey of velvet proteins in the fungal kingdom and beyond to reveal their distribution, protein size, and domain architecture. We then grouped the 21 major clades of velvet proteins in fungi based on the phylogenetic analysis and compared their conserved motifs and 3D structures. Altogether, our results suggest that velvet proteins are widely distributed in the fungal kingdom but also outside the kingdom. The velvet domain is highly conserved with three characteristic motifs and could combine with different functional domains, resulting in the diversity of velvet proteins. By analyzing the primary and 3D structures of various velvet domains across the fungal kingdom, we found that fungal velvet domains can be divided into two clans (VelB clan and VosA clan). Based on the structural comparison, we proposed that the velvet domain, together with the DNA-binding domains of the Rel, Runt, and STAT families sharing a similar β-sandwich fold, should belong to the same DNA-binding domain superfamily.
MATERIALS AND METHODS
Species selection and their sequence data
In this study, to address the diversity of velvet proteins in the fungal kingdom, the fungal genomic database of MycoCosm (https://mycocosm.jgi.doe.gov/mycocosm/home) (38) (2384 fungal genomes, accessed on 5 December 2022) covering the phyla Ascomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Cryptomycota, Microsporidia, Mucoromycota, and Zoopagomycota was queried. And then, the gene catalog proteins of these fungal species/strains were used for surveying the velvet distribution on the genomic scale.
The protein sequence databases of NCBI (51) and UniProt (52) were also accessed for searching velvet homologs outside the fungal kingdom.
Identification and annotation of velvet proteins
Velvet proteins are diverse but share a common and conserved velvet domain (8). Therefore, the rule for the identification of Velvet proteins is whether they contain velvet domains. In this study, the A. nidulans VeA velvet domain [position 34-231 of VeA protein GenBank: AAD42946.1 (13)] was used as a query to search for the homologs in the databases MycoCosm, NCBI, and UniProt by BlastP (53) with default parameters. Candidates close to the threshold were further confirmed in the NCBI conserved domain database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (43) for validating whether they hold full velvet domains and those with incomplete velvet domains were filtered. This approach is simple but highly effective because the velvet domain is highly conserved and unique and displays no sequence similarity to other known domains.
For annotation of the velvet candidates, they, together with the references A. nidulans VeA (GenBank: AAD42946.1), VelB (GenBank: ABQ17967.1), VelC (GenBank: ABQ17968.1), and VosA (GenBank: ABI51618.1), were subjected to phylogenetic analysis. The putative velvet proteins were classified into different velvet clades based on their phylogenetic relationship. The clades were named as the species taxonomic group followed by the velvet name. The clade taxonomic group is named by the first three letters of corresponding species taxonomic name. When a velvet clade contained a reference velvet, the clade was assigned to the reference velvet name. When there was a clear distinction between a velvet clade and the four references in phylogeny, the clade was assigned to a new member named as Velvet1, Velvet2, etc.
Phylogenetic analysis of velvet proteins or domains
The phylogenetic analysis was performed as follows. First, multiple alignments of the velvet proteins or domains were carried out by the MAFFT online service with its default parameters (54). Second, the multiple alignments were used to infer their trees. When the alignments had less than 200 sequences, they were submitted to the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/) for estimating the maximum likelihood tree with the best-fit model (55). When the alignments had more than 200 sequences, they were submitted to the T-REX web server (http://www.trex.uqam.ca/) for inferring phylogenetic trees with the best-fit method (56). Finally, the figures of phylogenetic trees were edited and generated by iTOL (https://itol.embl.de/) (57).
Analysis of characteristic motifs and residues of velvet domains
The characteristic motifs of the velvet domains were compared among different velvet clades to reveal their clade-shared or -specific conserved residues. First, multiple alignments of protein sequences within each clade were constructed using HMMER 3.1 (http://hmmer.org/) against the profile velvet hidden Markov model (https://www.ebi.ac.uk/interpro/entry/pfam/PF11754/) in InterPro (50). The residues assigned to match states that were conserved against the profile velvet hidden Markov model were reserved for constructing their consensus sequence logos.
Then, the consensus logos were generated from the alignments by WebLogo 3.7.12 (http://weblogo.threeplusone.com/create.cgi) for visualization of the conservation of the primary structure by plotting a stack of amino acids for each position (58, 59). The evolutionary conservation of each amino acid position in the alignment was determined using the ConSurf web-server (60, 61).
Survey of functional domains and motifs among velvet proteins
Putative functional domains of velvet proteins were surveyed among different velvet clades. The protein sequences were submitted to the NCBI conserved domain database (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) to annotate their functional domains (43).
Furthermore, NES of velvet domains were predicted using NESmapper 1.1 (62), and their NLS were mined using NLStradamus (63). Prediction of the subcellular localization of velvet domains was performed using WoLF PSORT targeting fungal species (64).
3D structure modeling and comparison
The 3D structure modeling of velvet domains was accomplished using AlphaFold v2.3.1 (65) pipelined in ColabFold v1.5.2 (66). Then, the 3D structures were displayed by the viewer Jmol (www.jmol.org). The comparison of protein structures was performed by PROMALS3D (67), the Dali server (68), and the Pairwise Structure Alignment server of PDB (https://www.rcsb.org/alignment). The search for similar structures of velvet domains against the database of Protein Data Bank (https://www.rcsb.org/) was performed in the Dali server (68) with the query structure of VosA velvet domain chain A (PDB ID: 4N6Q) (4). Meanwhile, the VosA velvet domain was also used as a query to search for similar structures in the NCBI protein structure database (https://www.ncbi.nlm.nih.gov/Structure/) using the Vector Alignment Search Tool Plus (69).
Statistical analysis
Boxplots of lengths of velvet proteins or domains were generated using OriginPro 8 (Massachusetts, USA) or the BoxPlotR (41). Two-group comparisons were performed using the unpaired t-test. P-values less than 0.05 were considered statistically significant, and those less than 0.001 were considered statistically highly significant.
ACKNOWLEDGMENTS
The work at UW-Madison is supported by the National Institute of Food and Agriculture, United States Department of Agriculture, Hatch project 7000326, and Food Research Institute of UW-Madison. This work at KNU was supported by the National Research Foundation of Korea (NRF) grant to H.-S.P. funded by the Korean government (NRF-2020R1C1C1004473) and a project to train professional personnel in biological materials by the Ministry of Environment.
Contributor Information
Hee-Soo Park, Email: phsoo97@knu.ac.kr.
Jae-Hyuk Yu, Email: jyu1@wisc.edu.
Miguel A. Penalva, Centro de Investigaciones Biologicas CSIC, Madrid, Spain
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03717-23.
Figures S1 to S7.
Summary of functional domains in the 21 velvet clades.
Top 300 similar structures of VosA velvet domain in the PDB database by the Dali server.
Similar structures of VosA velvet domain in the 3D macromolecular structures database of NCBI by VAST+.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Mafessoni F, Lachmann M, Gokhale CS. 2021. On the fitness of informative cues in complex environments. J Theor Biol 527:110819. doi: 10.1016/j.jtbi.2021.110819 [DOI] [PubMed] [Google Scholar]
- 2. Wingfield JC, Patrick Kelley J, Angelier F, Chastel O, Lei F, Lynn SE, Miner B, Davis JE, Li D, Wang G. 2011. Organism–environment interactions in a changing world: a mechanistic approach. J Ornithol 152:279–288. doi: 10.1007/s10336-011-0668-3 [DOI] [Google Scholar]
- 3. Leger MM, Ros-Rocher N, Najle SR, Ruiz-Trillo I, Baldauf S. 2022. Rel/NF-κB transcription factors emerged at the onset of opisthokonts. Genome Biol Evol 14:evab289. doi: 10.1093/gbe/evab289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed YL, Gerke J, Park H-S, Bayram Ö, Neumann P, Ni M, Dickmanns A, Kim SC, Yu J-H, Braus GH, Ficner R, Stock AM. 2013. The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-κB. PLoS Biol 11:e1001750. doi: 10.1371/journal.pbio.1001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghosh S, Hayden MS. 2012. Celebrating 25 years of NF-κB research. Immunol Rev 246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biancalana M, Natan E, Lenardo MJ, Fersht AR. 2021. NF-κB Rel subunit exchange on a physiological timescale. Protein Sci 30:1818–1832. doi: 10.1002/pro.4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torruella G, de Mendoza A, Grau-Bové X, Antó M, Chaplin MA, del Campo J, Eme L, Pérez-Cordón G, Whipps CM, Nichols KM, Paley R, Roger AJ, Sitjà-Bobadilla A, Donachie S, Ruiz-Trillo I. 2015. Phylogenomics reveals convergent evolution of lifestyles in close relatives of animals and fungi. Curr Biol 25:2404–2410. doi: 10.1016/j.cub.2015.07.053 [DOI] [PubMed] [Google Scholar]
- 8. Bayram O, Braus GH. 2012. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev 36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- 9. Sarikaya-Bayram Ö, Palmer JM, Keller N, Braus GH, Bayram Ö. 2015. One Juliet and four romeos: VeA and its methyltransferases. Front Microbiol 6:1. doi: 10.3389/fmicb.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu M-Y, Mead ME, Lee M-K, Neuhaus GF, Adpressa DA, Martien JI, Son Y-E, Moon H, Amador-Noguez D, Han K-H, Rokas A, Loesgen S, Yu J-H, Park H-S, Lin X. 2021. Transcriptomic, protein-DNA interaction, and metabolomic studies of VosA, VelB, and WetA in Aspergillus nidulans asexual spores. mBio 12:e03128-20. doi: 10.1128/mBio.03128-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thieme KG, Gerke J, Sasse C, Valerius O, Thieme S, Karimi R, Heinrich AK, Finkernagel F, Smith K, Bode HB, Freitag M, Ram AFJ, Braus GH, Copenhaver GP. 2018. Velvet domain protein VosA represses the zinc cluster transcription factor SclB regulatory network for Aspergillus nidulans asexual development, oxidative stress response and secondary metabolism. PLoS Genet 14:e1007511. doi: 10.1371/journal.pgen.1007511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Käfer E. 1965. Origins of translocations in Aspergillus nidulans. Genetics 52:217–232. doi: 10.1093/genetics/52.1.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Han K, Kim K, Han D, Jahng K, Chae K. 2002. The VeA gene activates sexual development in Aspergillus nidulans. Fungal Genet Biol 37:72–80. doi: 10.1016/s1087-1845(02)00029-4 [DOI] [PubMed] [Google Scholar]
- 14. Ni M, Yu J-H. 2007. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2:e970. doi: 10.1371/journal.pone.0000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH. 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504–1506. doi: 10.1126/science.1155888 [DOI] [PubMed] [Google Scholar]
- 16. Park H-S, Ni M, Jeong KC, Kim YH, Yu J-H, Harris S. 2012. The role, interaction and regulation of the velvet regulator VelB in Aspergillus nidulans. PLoS ONE 7:e45935. doi: 10.1371/journal.pone.0045935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park HS, Nam TY, Han KH, Kim SC, Yu JH. 2014. VelC positively controls sexual development in Aspergillus nidulans. PLoS One 9:e89883. doi: 10.1371/journal.pone.0089883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato N, Brooks W, Calvo AM. 2003. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell 2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spröte P, Brakhage AA. 2007. The light-dependent regulator velvet A of Aspergillus nidulans acts as a repressor of the penicillin biosynthesis. Arch Microbiol 188:69–79. doi: 10.1007/s00203-007-0224-y [DOI] [PubMed] [Google Scholar]
- 20. Sarikaya Bayram Ö, Bayram Ö, Valerius O, Park HS, Irniger S, Gerke J, Ni M, Han K-H, Yu J-H, Braus GH, Brakhage AA. 2010. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet 6:e1001226. doi: 10.1371/journal.pgen.1001226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim M-J, Lee M-K, Pham HQ, Gu MJ, Zhu B, Son S-H, Hahn D, Shin J-H, Yu J-H, Park H-S, Han K-H. 2020. The velvet regulator VosA governs survival and secondary metabolism of sexual spores in Aspergillus nidulans Genes (Basel) 11:103. doi: 10.3390/genes11010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Myung K, Guse D, Donkin B, Proctor RH, Grayburn WS, Calvo AM. 2006. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Mol Microbiol 62:1418–1432. doi: 10.1111/j.1365-2958.2006.05447.x [DOI] [PubMed] [Google Scholar]
- 23. Dreyer J, Eichhorn H, Friedlin E, Kürnsteiner H, Kück U. 2007. A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Appl Environ Microbiol 73:3412–3422. doi: 10.1128/AEM.00129-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu D, Oide S, Zhang N, Choi MY, Turgeon BG, Tyler B. 2012. ChLae1 and ChVel1 regulate T-toxin production, virulence, oxidative stress response, and development of the maize pathogen Cochliobolus heterostrophus. PLoS Pathog 8:e1002542. doi: 10.1371/journal.ppat.1002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laskowski-Peak MC, Calvo AM, Rohrssen J, Smulian AG. 2012. VeA1 is required for cleistothecial formation and virulence in Histoplasma capsulatum. Fungal Genet Biol 49:838–846. doi: 10.1016/j.fgb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 26. Choi Y-E, Goodwin SB. 2011. MVE1, encoding the velvet gene product homolog in Mycosphaerella graminicola, is associated with aerial mycelium formation, melanin biosynthesis, hyphal swelling, and light signaling. Appl Environ Microbiol 77:942–953. doi: 10.1128/AEM.01830-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bayram O, Krappmann S, Seiler S, Vogt N, Braus GH. 2008. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet Biol 45:127–138. doi: 10.1016/j.fgb.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 28. Bayram ÖS, Dettmann A, Karahoda B, Moloney NM, Ormsby T, McGowan J, Cea-Sánchez S, Miralles-Durán A, Brancini GTP, Luque EM, Fitzpatrick DA, Cánovas D, Corrochano LM, Doyle S, Selker EU, Seiler S, Bayram Ö. 2019. Control of development, secondary metabolism and light-dependent carotenoid biosynthesis by the velvet complex of Neurospora crassa. Genetics 212:691–710. doi: 10.1534/genetics.119.302277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I, Kürnsteiner H, Kück U. 2010. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot Cell 9:1236–1250. doi: 10.1128/EC.00077-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karakkat BB, Gold SE, Covert SF. 2013. Two members of the Ustilago maydis velvet family influence teliospore development and virulence on maize seedlings. Fungal Genet Biol 61:111–119. doi: 10.1016/j.fgb.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 31. Ojeda-López M, Chen W, Eagle CE, Gutiérrez G, Jia WL, Swilaiman SS, Huang Z, Park H-S, Yu J-H, Cánovas D, Dyer PS. 2018. Evolution of asexual and sexual reproduction in the aspergilli. Stud Mycol 91:37–59. doi: 10.1016/j.simyco.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Etxebeste O, Otamendi A, Garzia A, Espeso EA, Cortese MS. 2019. Rewiring of transcriptional networks as a major event leading to the diversity of asexual multicellularity in fungi. Crit Rev Microbiol 45:548–563. doi: 10.1080/1040841X.2019.1630359 [DOI] [PubMed] [Google Scholar]
- 33. Park H-S, Man Yu Y, Lee M-K, Jae Maeng P, Chang Kim S, Yu J-H. 2015. Velvet-mediated repression of β-glucan synthesis in Aspergillus nidulans spores. Sci Rep 5:10199. doi: 10.1038/srep10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park H-S, Yu J-H. 2016. Velvet regulators in Aspergillus spp. Microbiol Biotechnol Lett 44:409–419. doi: 10.4014/mbl.1607.07007 [DOI] [Google Scholar]
- 35. Schumacher J, Simon A, Cohrs KC, Traeger S, Porquier A, Dalmais B, Viaud M, Tudzynski B. 2015. The VELVET complex in the gray mold fungus Botrytis cinerea: impact of BcLAE1 on differentiation, secondary metabolism, and virulence. Mol Plant Microbe Interact 28:659–674. doi: 10.1094/MPMI-12-14-0411-R [DOI] [PubMed] [Google Scholar]
- 36. Kopke K, Hoff B, Bloemendal S, Katschorowski A, Kamerewerd J, Kück U. 2013. Members of the Penicillium chrysogenum velvet complex play functionally opposing roles in the regulation of penicillin biosynthesis and Conidiation. Eukaryot Cell 12:299–310. doi: 10.1128/EC.00272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Höfer AM, Harting R, Aßmann NF, Gerke J, Schmitt K, Starke J, Bayram Ö, Tran V-T, Valerius O, Braus-Stromeyer SA, Braus GH, Freitag M. 2021. The velvet protein Vel1 controls initial plant root colonization and conidia formation for xylem distribution in verticillium wilt. PLoS Genet 17:e1009434. doi: 10.1371/journal.pgen.1009434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, Riley R, Salamov A, Zhao X, Korzeniewski F, Smirnova T, Nordberg H, Dubchak I, Shabalov I. 2014. Mycocosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res 42:D699–D704. doi: 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Money NP. 2016. Chapter 1 - fungal diversity, p 1–36. In Watkinson SC, Boddy L, Money NP (ed), The fungi, 3rd ed. Academic Press, Boston. [Google Scholar]
- 40. Cho H-J, Son S-H, Chen W, Son Y-E, Lee I, Yu J-H, Park H-S. 2022. Regulation of conidiogenesis in Aspergillus flavus Cells 11:2796. doi: 10.3390/cells11182796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spitzer M, Wildenhain J, Rappsilber J, Tyers M. 2014. Boxplotr: a web tool for generation of box plots. Nat Methods 11:121–122. doi: 10.1038/nmeth.2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen X, Xu J, Huang B, Li J, Wu X, Ma L, Jia X, Bian X, Tan F, Liu L, Chen S, Li X. 2011. A sub-pathway-based approach for identifying drug response principal network. Bioinformatics 27:649–654. doi: 10.1093/bioinformatics/btq714 [DOI] [PubMed] [Google Scholar]
- 43. Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. 2020. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48:D265–D268. doi: 10.1093/nar/gkz991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suga H, Chen Z, de Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, Carr M, Kerner P, Vervoort M, Sánchez-Pons N, Torruella G, Derelle R, Manning G, Lang BF, Russ C, Haas BJ, Roger AJ, Nusbaum C, Ruiz-Trillo I. 2013. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat Commun 4:2325. doi: 10.1038/ncomms3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I. 2020. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020:baaa062. doi: 10.1093/database/baaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sullivan JC, Kalaitzidis D, Gilmore TD, Finnerty JR. 2007. Rel homology domain-containing transcription factors in the cnidarian Nematostella vectensis. Dev Genes Evol 217:63–72. doi: 10.1007/s00427-006-0111-6 [DOI] [PubMed] [Google Scholar]
- 47. Jia S, Flores-Saaib RD, Courey AJ. 2002. The dorsal Rel homology domain plays an active role in transcriptional regulation. Mol Cell Biol 22:5089–5099. doi: 10.1128/MCB.22.14.5089-5099.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. 2019. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front Oncol 9:48. doi: 10.3389/fonc.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin T-C. 2022. Runx1 and cancer. Biochim Biophys Acta Rev Cancer 1877:188715. doi: 10.1016/j.bbcan.2022.188715 [DOI] [PubMed] [Google Scholar]
- 50. Monzon V, Paysan-Lafosse T, Wood V, Bateman A. 2022. Reciprocal best structure hits: using alphafold models to discover distant homologues. Bioinform Adv 2:vbac072. doi: 10.1093/bioadv/vbac072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, Wang J, Williams R, Trawick BW, Pruitt KD, Sherry ST. 2022. Database resources of the national center for biotechnology information. Nucleic Acids Res 50:D20–D26. doi: 10.1093/nar/gkab1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. UniProt C. 2023. Uniprot: the universal protein knowledgebase in 2023. Nucleic Acids Res 51:D523–D531. doi: 10.1093/nar/gkac1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 54. Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. doi: 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. doi: 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boc A, Diallo AB, Makarenkov V. 2012. T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res 40:W573–W579. doi: 10.1093/nar/gks485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Letunic I, Bork P. 2021. Interactive tree of life (iTOL) V5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. doi: 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider TD, Stephens RM. 1990. Sequence logos - a new way to display consensus sequences. Nucleic Acids Res 18:6097–6100. doi: 10.1093/nar/18.20.6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. Weblogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ben Chorin A, Masrati G, Kessel A, Narunsky A, Sprinzak J, Lahav S, Ashkenazy H, Ben-Tal N. 2020. ConSurf-DB: an accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci 29:258–267. doi: 10.1002/pro.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goldenberg O, Erez E, Nimrod G, Ben-Tal N. 2009. The ConSurf-DB: pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Res 37:D323–D327. doi: 10.1093/nar/gkn822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kosugi S, Yanagawa H, Terauchi R, Tabata S. 2014. Nesmapper: accurate prediction of Leucine-rich nuclear export signals using activity-based profiles. PLoS Comput Biol 10:e1003841. doi: 10.1371/journal.pcbi.1003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nguyen Ba AN, Pogoutse A, Provart N, Moses AM. 2009. NLStradamus: a simple hidden markov model for nuclear localization signal prediction. BMC Bioinformatics 10:202. doi: 10.1186/1471-2105-10-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. 2007. WoLF PSORT: protein localization predictor. Nucleic Acids Res 35:W585–W587. doi: 10.1093/nar/gkm259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. 2021. Highly accurate protein structure prediction with alphafold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. 2022. ColabFold: making protein folding accessible to all. Nat Methods 19:679–682. doi: 10.1038/s41592-022-01488-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pei J, Tang M, Grishin NV. 2008. Promals3D web server for accurate multiple protein sequence and structure alignments. Nucleic Acids Res 36:W30–W34. doi: 10.1093/nar/gkn322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Holm L. 2022. Dali server: structural unification of protein families. Nucleic Acids Res 50:W210–W215. doi: 10.1093/nar/gkac387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Madej T, Lanczycki CJ, Zhang D, Thiessen PA, Geer RC, Marchler-Bauer A, Bryant SH. 2014. MMDB and VAST+: tracking structural similarities between macromolecular complexes. Nucleic Acids Res 42:D297–D303. doi: 10.1093/nar/gkt1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S7.
Summary of functional domains in the 21 velvet clades.
Top 300 similar structures of VosA velvet domain in the PDB database by the Dali server.
Similar structures of VosA velvet domain in the 3D macromolecular structures database of NCBI by VAST+.