Extended Data Fig. 10. Proposed mechanism of length-dependent activation (LDA) and mechanosensing (MS) in cardiac thick filament involving titin, cMyBP-C and myosin tails (zoom for detail).
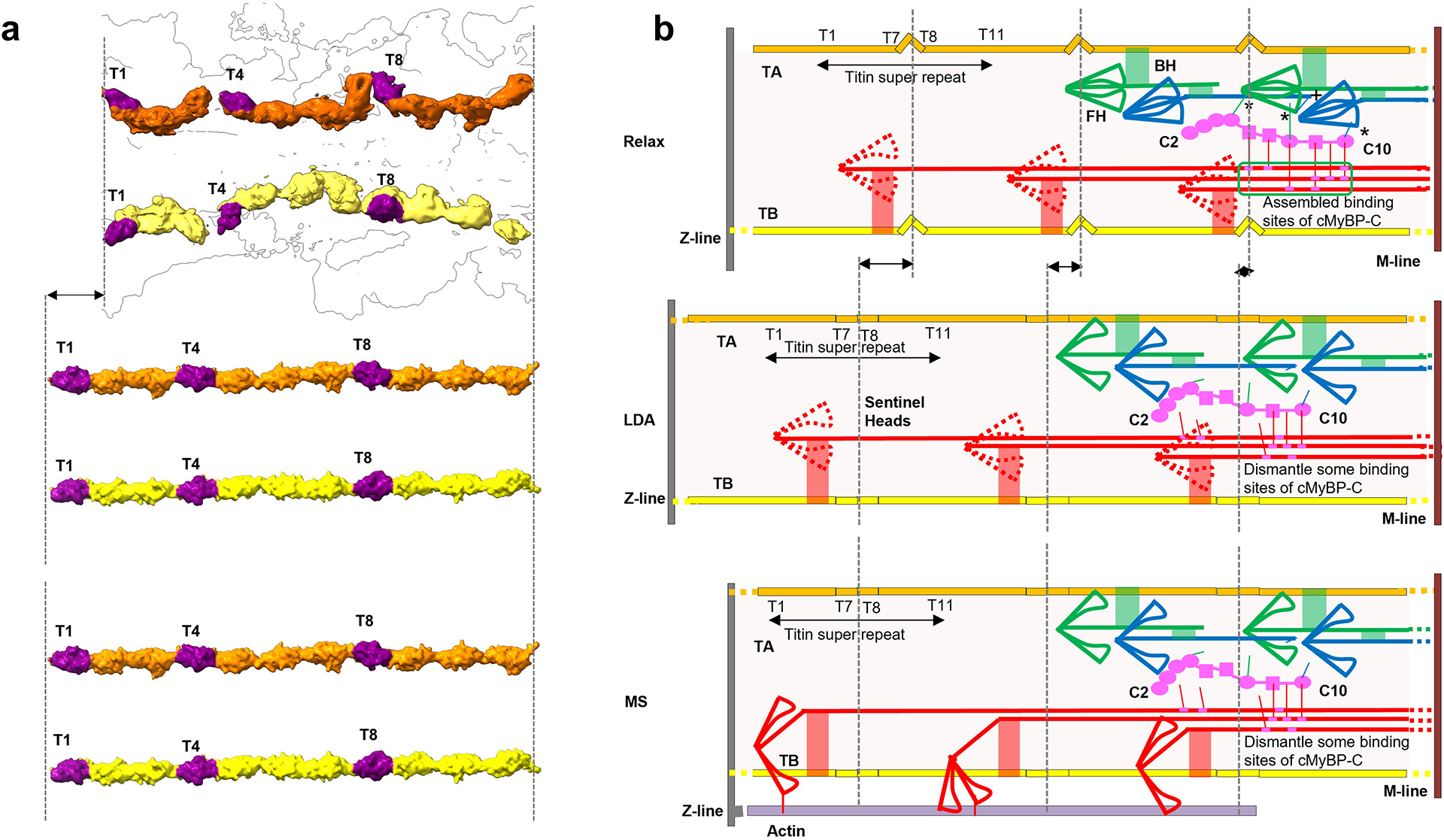
Our reconstruction reveals connections of titin to myosin and myosin to cMyBP-C, that may underlie LDA and MS. a, b. Upper (relaxed): In relaxed state (right), TB and TA are kinked as in our reconstruction (left). Myosin heads form IHMs; cMyBP-C binds to docking site on CrD tails (green box; ED Fig. 8) and to CrH and CrT heads (asterisks) (Fig. 5c–f); and TB and TA interact with myosin tails (red, green connections). In addition, CrH FH CM loop binds to TaT tail (“+” in b (upper); Fig. 6g, h). Middle (LDA): Elongation of the sarcomere at end-diastole (right) stretches TB and TA, reducing the kinks (left), supported by an increase in the spacing of the 39 Å X-ray reflection upon stretch74; see ED Figs. 3g, 6c). Tension-induced translation of titin domains pulls on connected myosin tails, partially dismantling cMyBP-C binding sites on CrD tails and on CrH and CrT heads. Loss of the stabilizing influence of cMyBP-C on CrH and CrT, augmented by weakening of the CrH FH CM loop-TaT interaction (“+” in b, upper) under tension, releases heads for actin interaction, accounting for progressive enhanced force of systole that follows corresponding sarcomere length increase (= LDA). Lower (MS): In mechanosensing (right), CrD heads that performed a “sentinel” role for detecting thin filament activation75,76 in relaxed and LDA states, are now active and produce tension by interacting with actin (right). This stretches titin in a similar way to LDA (left), with similar consequences, enhancing contractility. In addition to the above cMyBP-C interactions, there are also CrD tail interactions with CrH FH and CrT FH, which could stabilize these IHMs (Fig. 6j, k, m). These interactions would also be broken upon sliding of CrD tails, contributing to LDA and MS.
