Extended Data Fig. 3. Validation of reconstruction.
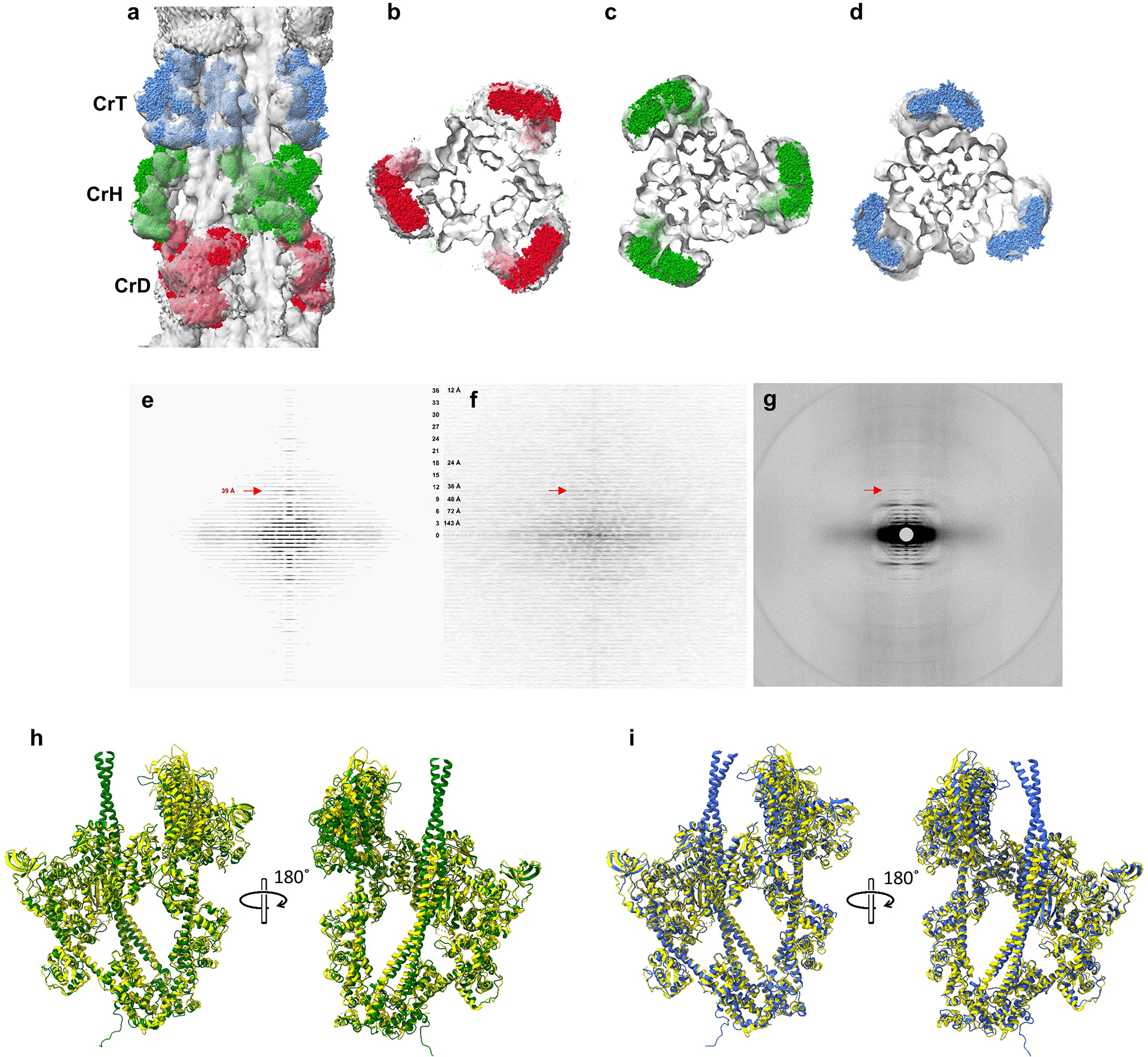
a-d, We compared our reconstruction with X-ray diffraction data, which provide information on filament structure in the lattice of intact muscle. a, Longitudinal, and b-d, transverse views of our cardiac thick filament reconstruction at the 3 crowns in the 430 Å repeat (gray map), fitted with thick filament atomic model (CrH, green; CrT, blue; CrD, red) based on X-ray diffraction data22 combined with previous negative stain reconstructions17,18. The excellent fit of the X-ray model to the cryo-EM map, especially the radial positioning of the heads (center-of-mass ~135 Å from the filament axis22), suggests that the reconstruction is close to the structure in intact muscle, and strongly supports its validity. The previous negative stain reconstructions had suggested a head center-of-mass at ~95 Å radius (~40 Å less than the cryo-reconstruction), thought to be due to radial collapse of heads during drying of negative stain22—not an issue with frozen-hydrated specimens. e-f, We also compared an averaged power spectrum of the reconstruction (rotated at intervals of 10° from 0 to 110°) (e) with an averaged power spectrum of selected filaments used in the reconstruction (f). The similarity of the two power spectra, both extending to the 36th order of the 430 Å repeat (12 Å), supports the validity of the reconstruction. g, Wide-angle X-ray diffraction pattern of intact, relaxed porcine cardiac muscle at same scale (courtesy of Drs. Tom Irving and Weikang Ma, unpublished data) shows similar myosin layer lines at low angles, further suggesting that our structure is similar to that in intact muscle. A key feature of all patterns is the prominent 39 Å reflection (11th order of 430 Å, red arrows). Previous X-ray studies have suggested that this may be due to titin72. Our reconstruction reveals titin unambiguously (see text and ED Fig. 5), and we show that the reflection arises from the kinking of its elongated structure, allowing 11 domains to fit into the 430 Å repeat (ED Fig. 6c). h, i. We also compared our native filament IHMs (CrH and CrT) with the cryo-EM structure of a recombinant IHM containing 15 heptads of tail (PDB 8ACT46). 8ACT (yellow) was superimposed on CrH (green) and CrT (blue) (h, i, respectively). Superposition was performed by matching BHs (Matchmaker in ChimeraX) to reveal how the FH is positioned with respect to the BH in each case. The 3 different IHMs showed strong similarity. However, the two filament IHMs are non-identical (Fig. 2h), due to different interactions with cMyBP-C and the backbone in the environment of the native filament (Fig. 5c–f, Fig. 6g–m). Although the fit of 8ACT to the filament IHMs was not perfect, its individual blocked and free heads showed excellent correspondence to the respective CrT and CrH heads. CrH BH and 8ACT BH RMSD between 618 pruned atom pairs was 1.179 Å, and across all 867 pairs was 2.148 Å; CrH FH and 8ACT FH RMSD between 592 pruned atom pairs was 1.268 Å, and across all 851 pairs was 3.371 Å; CrT BH and 8ACT BH RMSD between 554 pruned atom pairs was 1.247 Å, and across all 867 pairs was 2.328 Å; CrT FH and 8ACT FH RMSD between 531 pruned atom pairs was 1.287 Å, and across all 851 pairs was 2.526 Å. We conclude that 8ACT is an excellent model for the two filament IHMs, its individual heads matching essentially perfectly with the individual free and blocked heads of CrT and CrH, and the whole IHM being close to the CrT and CrH whole IHMs (overall closer to CrH). The small difference between the isolated IHM and its counterparts in the filament is likely due to the absence in the isolated molecule of interactions of the FH and BH that occur with cMyBP-C and the filament backbone in the native filament. RMSD of Cα was calculated with the ChimeraX Matchmaker command using FH and BH separately.
