Figure 8.
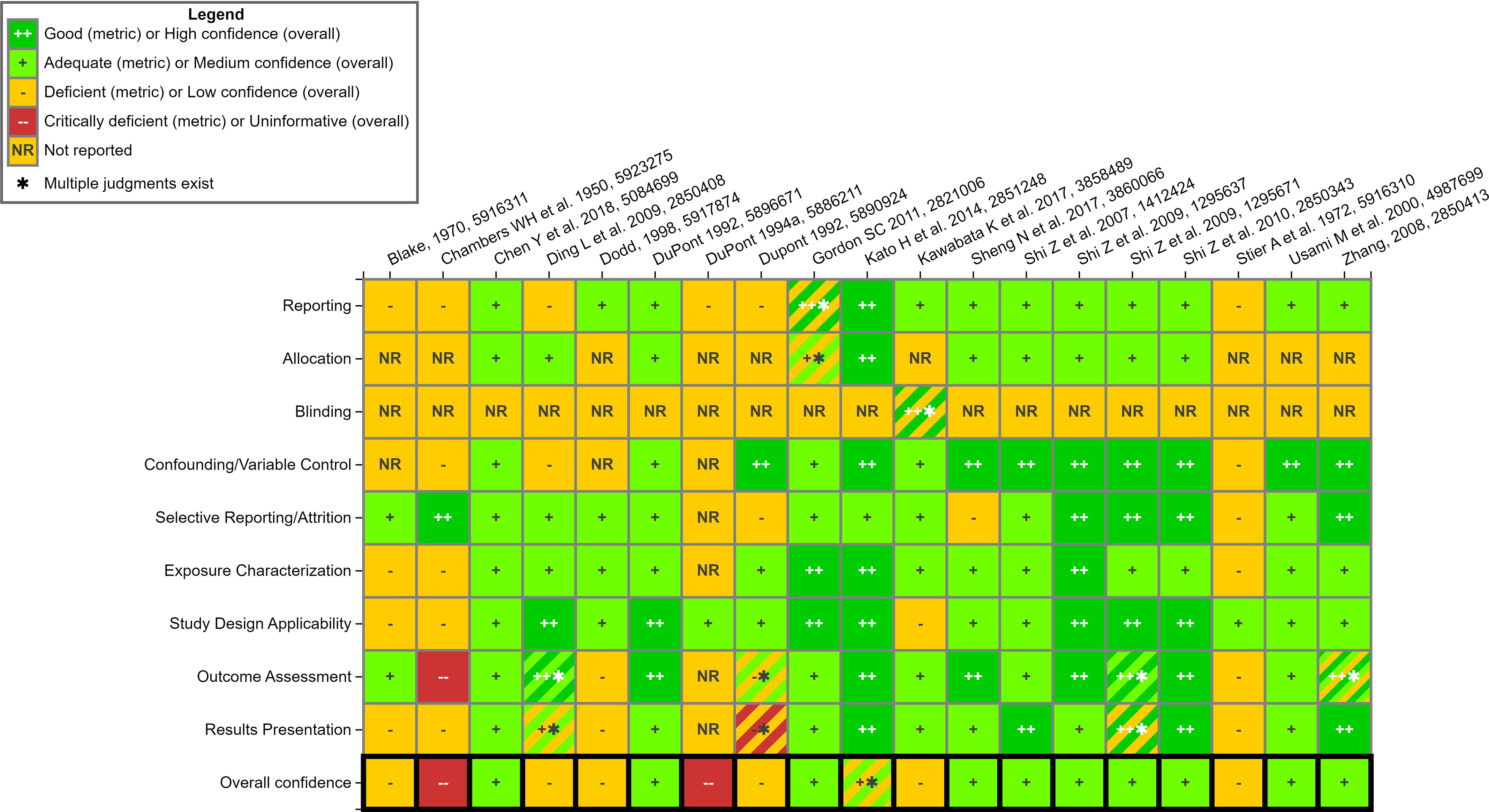
Study evaluation for mammalian bioassay studies- non-ECHA reports. This is a thumbnail image of the interactive visual (https://hawc.epa.gov/summary/visual/assessment/100500256/Animal-SQE-Heatmap_nonECHA/) accessed on 3 November 2023.67 The study evaluation approach follows standard methods that are used in systematic evidence maps developed by the US EPA27,28,32 and have only been adjusted, where appropriate, for the specific needs of this SEM. A full download of detailed evaluation summaries is available through HAWC, or in Excel Table S11. Note: ECHA, European Chemicals Agency; HAWC, Health Assessment Workspace Collaborative; SEM, Systematic Evidence Map.
