Figure 5. Molecular chaperones suppress phase separation.
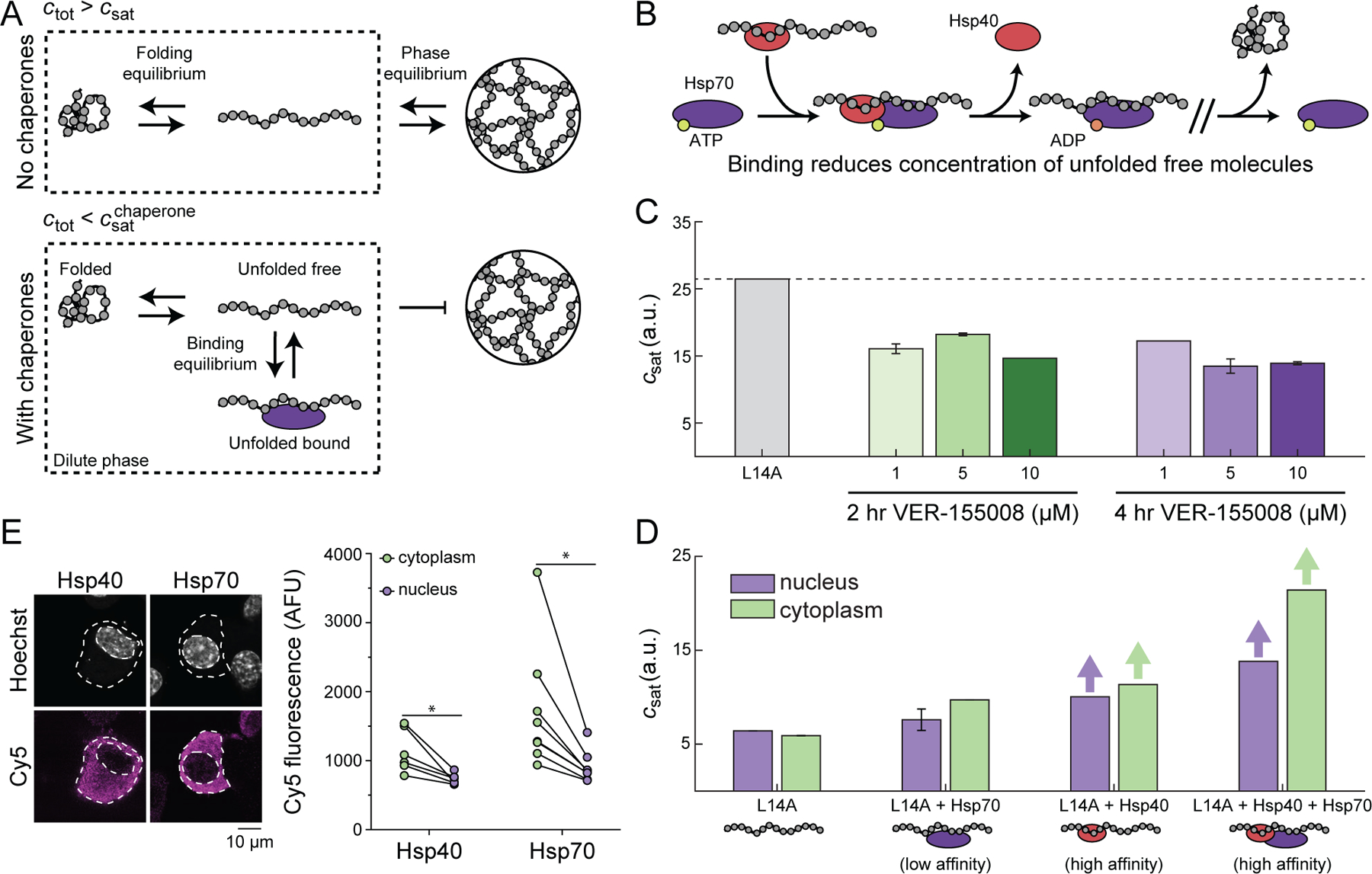
(A) In the absence of chaperones, the dilute phase consists of folded and unfolded barnase. There exists a phase equilibrium when the total concentration of barnase, ctot, is greater than csat. In the presence of chaperones, barnase in the dilute phase consists of three dominant states: folded, unfolded free, and unfolded bound to chaperones. At the same total concentration of barnase as in the absence of chaperones, barnase cannot phase separate because ctot is less than the saturation concentration needed in the presence of chaperones, given that chaperone binding reduces the concentration of free unfolded barnase. (B) Basic model for chaperone function. Hsp40 binds the unfolded molecule and forms a ternary complex with Hsp70 in the ATP-bound state. ATP hydrolysis leads to the release of Hsp40 and the formation of a high affinity complex between Hsp70 and the unfolded substrates. (C) csat for the L14A barnase-optoDroplet variant construct transiently transfected in Neuro2A cells in the absence or presence of different dosages of the Hsp70 inhibitor VER-155008. Dashed line corresponds to the csat of L14A in the absence of the inhibitor. Error bars denote the standard deviation from 50 bootstrapped trials. (D) csat for the L14A barnase-optoDroplet variant construct in the absence or presence of overexpressed chaperones. Bars with arrows indicate a csat value could not be extracted for these systems and must be at least above the value of the bar. Error bars denote the standard deviation from 50 bootstrapped trials. (E) Confocal images of Neuro2a cells transfected with V5-tagged DNAJB1 (Hsp40) or HSPA1A (Hsp70). Cells were stained by immunofluorescence for the V5-tag (Cy5) and the nucleus was stained with Hoechst 33342. Graphs show quantitation of immunofluorescence. Data shown as paired samples from individual cells. Paired t-test results shown; * p<0.05. See also Figure S4 and Table S4.
