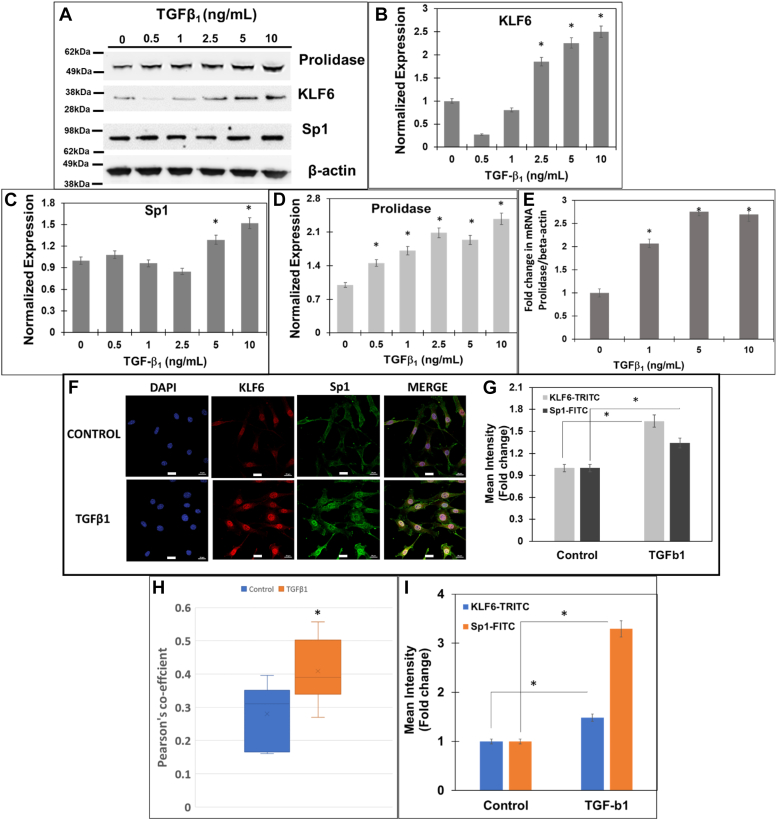
Figure 4.
TGF-β1increases expression of prolidase concurrent with increased KLF6 and Sp1 in mouse fibroblasts. NIH3T3 cells were serum-starved for 6 h and treated with TGF-β1 in a concentration and time-dependent manner. After treatment total RNA was isolated for mRNA quantification and cell lysates were prepared for protein measurements. Prolidase mRNA expression was measured by qPCR and normalized to β-actin. Equal protein amounts of cell lysates were subjected to immunoblot analyses to measure protein expression. A, representative Immunoblot showing expression of prolidase, KLF6, Sp1, and β-actin. Densitometry analyses of (B) KLF6, (C) Sp1, and (D) prolidase expression normalized to β-actin. E, the fold change in prolidase mRNA expression with increasing concentration of TGF-β1 treatment is represented as treated vs untreated cells based on ΔΔCt values. F–I, confocal microscopy analysis of KLF6 and Sp1 in NIH3T3 cells after treatment with TGF-β1. Following treatment cells were fixed, permeabilzed, and stained with blue staining of nuclei with DAPI, anti-KLF6 (red), and anti-Sp1 (green). F, representative confocal images showing the expression of KLF6 and Sp1 and their colocalization (merge). Scale bars: 20 μm. G, the calculated mean fluorescence intensity values show the increase in expression of both KLF6 and Sp1 following TGF-β1 treatment as compared to control cells. H, Pearson’s coefficient shows the co-localization of KLF6 and Sp1 following TGF-β1 treatment as compared to control cells. I, nuclear co-localization of KLF6 and Sp1 following TGF-β1 treatment as compared to control cells. Data are mean values of three independent experiments with error bars representing SEM. ∗ represents a p value of < 0.05 for the statistical comparison of (B–E and G–I) untreated vs TGF-β1 treated samples.