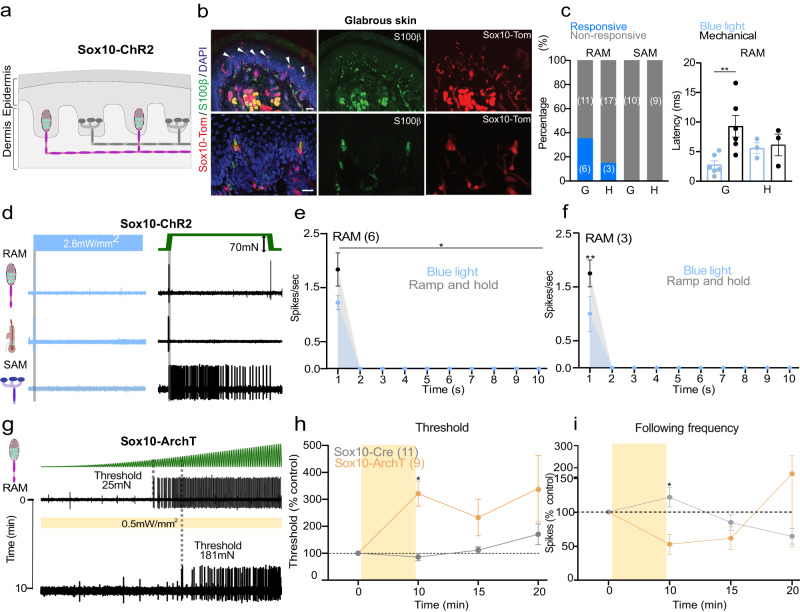
Fig. 3. Sensory Sox10+ Schwann cells in the Meissner’s corpuscle are required for vibration sensing.
a Schematic diagram of Sox10+ cells in the RAM but not in SAM fibers in the skin. b Immunofluorescence showing recombination in glial cells of Meissner corpuscles in Sox10-TOM mice. Immunohistochemistry for TOMATO (recapitulating Sox10 expression) and S100β to label glial cells in the corpuscle sensory ending. Upper panels show a low magnification image of the mice footpads with arrows pointing to Meissner corpuscles. Lower panels show higher magnification images. Scale bar: 50 μm (upper panels) and 20 μm (lower panels). This experiment was repeated at least 6 times. c On the left, total number of mechanoreceptors (RAM and SAM) recorded from the hairy (h) or glabrous skin (g) in Sox10-ChR2 mice, showing proportions of light responsive (blue) and non-responsive mechanoreceptors (gray). On the right, first spike latencies for RAMs comparing optogenetic activation of Schwann cells and mechanical activation of the same afferent during the ramp phase. RAMs recorded from Sox10-ChR2 mice respond faster to light stimulation than to ramp indentation applied at 15 mm/s via a piezo actuator (unpaired t-test, P = 0.007). d Example of spiking from RAMs and SAMs exposed to blue light compared to a mechanical stimulus recorded from glabrous or hairy skin. e, f Mean RAM spiking activity plotted in 1 s bins from Sox10-ChR2 mice during 10 s of blue light or mechanical stimulation from glabrous (e) (n = 6) or hairy skin (f), (n = 3). g Mechanoreceptor spiking rates in response to 20 Hz vibration stimulus before and after optogenetic inhibition of Schwann cells. Top, RAM representative trace; Bottom, the same unit 10 min after yellow light exposure. h Mechanical threshold for first spike for units recorded in Sox10-ArchT (n = 11) and control mice (n = 9). An increase in the force necessary to evoke the first action potential was observed in RAMs recorded from Sox10-ArchT mice, Two-way ANOVA, P = 0.0383, Bonferroni’s multiple comparisons test (two sided). i The following frequency decreased after yellow light stimulation in Sox10-ArchT+ mice at 10 min and this was statistically significant (Two-way ANOVA, P = 0.047, Bonferroni’s multiple comparisons test). Data are presented as mean values ± s.e.m. Source data are provided as a Source Data file.