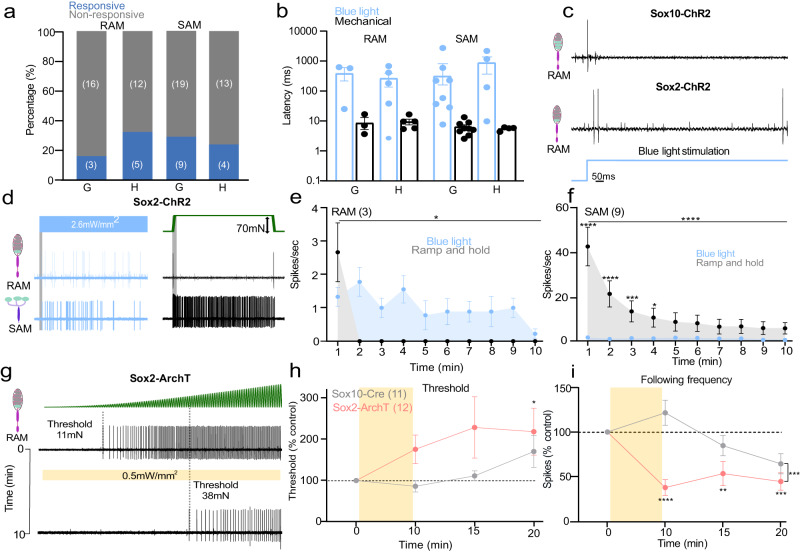
Fig. 5. Sensory Sox2+ Schwann cells in the Meissner’s corpuscle are required for vibration sensing.
a The proportion of mechanoreceptors (RAM and SAM) recorded from the hairy (H) or glabrous skin (G) in Sox2-ChR2 mice showing activation by blue light (blue), non-responsive (gray). b First spike latencies for RAMs comparing optogenetic activation of Schwann cells and mechanical activation of the same afferent during the ramp phase. RAMs recorded from Sox2-ChR2 mice much slower to blue stimulation than to ramp indentation applied at 15 mm/s via a piezo actuator (two sided unpaired t-test, P = 0.007). c Example of spiking from RAMs exposed to blue light from Sox2-ChR2 mice (top) and Sox2-ChR2 mice (bottom), note long latencies in the latter case. d Example traces of spiking from RAMs (top) or SAMs (bottom) exposed to blue light from Sox2-Chr2 mice compared to the response to a mechanical stimulus (right) in glabrous skin. e Mean spiking activity plotted in 1 s bins during 10 s of blue light or mechanical stimulation of RAMs in glabrous skin and (f) of SAMs in glabrous skin in Sox2-ChR2 mice. g Mechanoreceptor spiking rates in response to 20 Hz vibration stimulus before and after optogenetic inhibition of Sox2+ Schwann cells. Top, RAM representative trace; Bottom, the same unit 10 min after yellow light exposure. h Mechanical threshold for first spike for Sox2-ArchT and control mice. An increase in the force necessary to evoke the first action potential was observed in RAMs recorded from Sox10-ArchT mice (Two-way ANOVA, P = 0.0383, Bonferroni’s multiple comparisons test). i The following frequency decreased after yellow light stimulation in Sox2-ArchT+ mice at 10 min and this was statistically significant (two-way ANOVA, P = 0.047, Bonferroni’s multiple comparisons test). Data are presented as mean values ± s.e.m. Source data are provided as a Source Data file.