Table 2.
Typical electrocatalysts recently developed for homogeneous CO2ER
| Types | Molecular structure | Main product | Potential | Faradic efficiency | Reference |
|---|---|---|---|---|---|
| Ir-based complexes84 | 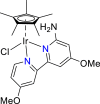 |
HCOO− | −1.80 V vs. (Ag/AgCl) | 38% | Sypaseuth et al.84 |
| Ni-based complexes85 | 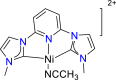 |
CO | −1.77 V vs. (SHE) | 34% | Sheng et al.85 |
| Pd-based complex86 | 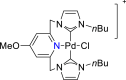 |
CO | −1.60 V vs. (Ag/AgNO3) | 85% | Therrien and Wolf86 |
| Re-based complex87 | 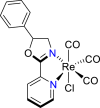 |
CO | −2.80 V vs. (Fc/Fc+) | 30-55% | Nganga et al.87 |
| Co-based complex62 | 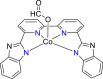 |
HCOO− | −2.32 V vs. (Fc/Fc+) | 68% | Tsubonouchi et al.62 |
| Mn-based complex88 | 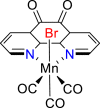 |
CO | −2.24 V vs. (Fc/Fc+) | 30-60% | Stanbury et al.88 |
| Ru-based complex89 | 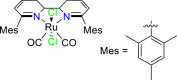 |
CO | −2.20 V vs. (Fc/Fc+) | 95% | Machan et al.89 |
| Non-metal ionic liquid complex90 | 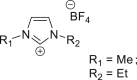 |
C2O42− | −2.60 V vs. (Fc/Fc+) | 39.3% | Rosen et al.90 |
The main products, potential, and Faradic efficiency for the corresponding electrocatalyst are also listed.
