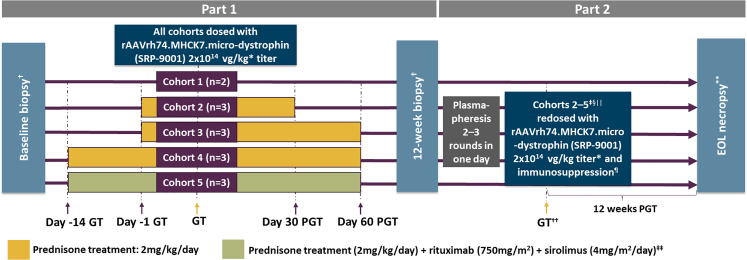
Figure 1.
Study design
∗Supercoiled standard qPCR titer method. †Biopsy collected from gastrocnemius muscle. ‡One nonhuman primate (NHP) did not undergo plasmapheresis, because of a lack of antibody response to AAVrh74 (adeno-associated virus rhesus isolate serotype 74). §One NHP did not undergo plasmapheresis, because of poor vascular access. ||Cohort 5 did not undergo plasmapheresis, because of incompatibility with previous treatment with rituximab. ¶All NHPs received prednisone (2 mg/kg/day) from 1 day pre-redosing to 30 days post-redosing with delandistrogene moxeparvovec. ∗∗End-of-life (EOL) necropsy collected from gastrocnemius, heart, and diaphragm. ††Immediately post-plasmapheresis, the NHPs were disconnected from the apheresis unit and systemically redosed with delandistrogene moxeparvovec. ‡‡Sirolimus was started 3 days prior to the first GTT dose and was continued until three weeks after the second dose. NHPs received one dose of rituximab 14 and 7 days prior to the first GTT dose, as well as one dose on the day of GTT dosing and one dose prior to redosing. GT, gene transfer; PGT, post-gene therapy.