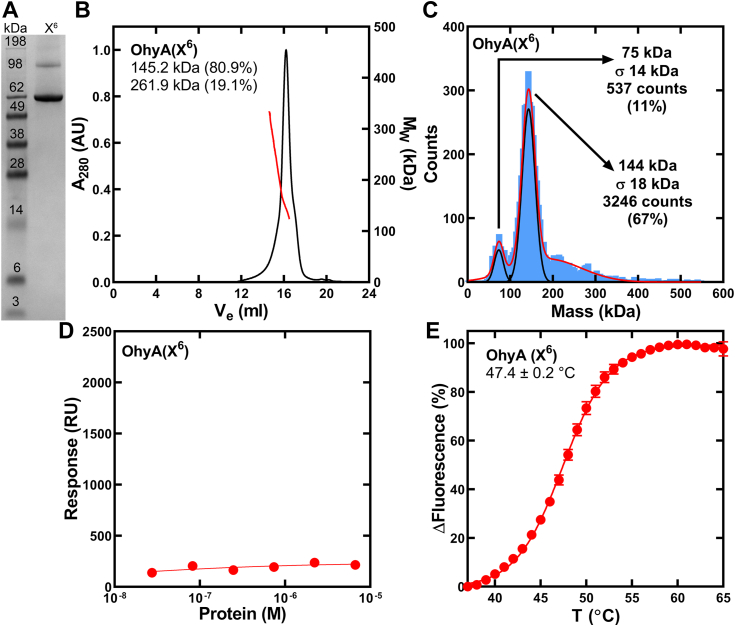
Figure 12.
Preparation and characterization of OhyA(X6).A, OhyA(X6) was a 65-kDa monomer and was >95% pure based on denaturing gel electrophoresis. B, His-tagged OhyA(X6) was purified by Ni2+-affinity chromatography followed by gel filtration chromatography on Superose 6 Increase 10/300 Gl and multi-angle light scattering (SEC-MALS). The molar mass distribution (red line) is superimposed on a chromatogram of A280versus elution volume (black line). SEC-MALS measured OhyA predominantly migrates as a 145-kDa dimer. C, mass photometry measured OhyA remains a 144-kDa dimer in dilute concentrations. D, surface plasmon resonance determination (n = 3 technical replicates) of DOPG/DOPC liposome binding affinity of OhyA(X6). The data from three independent titrations were fit to the Hill equation. Mean ± S.D. E, protein thermal denaturation analysis was used to determine the structural integrity of OhyA(X6). Assays (n = 5 technical replicates) contained 1 mg/ml OhyA(X6). The data are fit to the Boltzmann equation. Mean ± S.D.