FIG. 1. TubULAR is a toolkit for tracking dynamic surfaces such as visceral organs.
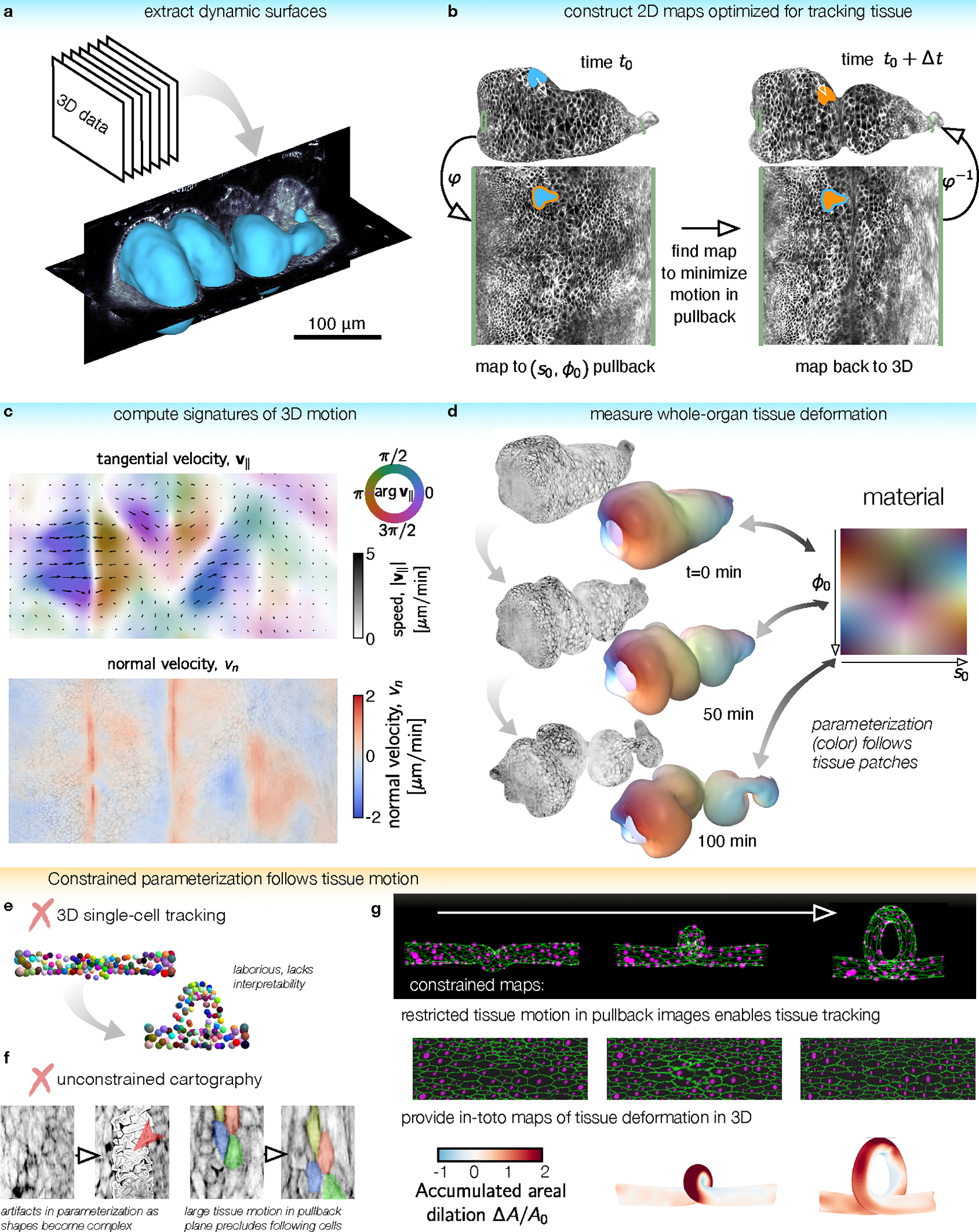
a, TubULAR first extracts dynamic surfaces of interest from volumetric datasets, here shown for the Drosophila midgut. b, Constrained parameterization of the whole surface facilitates tracking tissue motion. Mapping the surface at a reference timepoint to the plane defines a material coordinate system, . Pullback images of subsequent timepoints are optimized to be nearly stationary in the parameterization space. 3D tissue velocities (white arrow) are obtained by linking the 3D positions of each material coordinate across timepoints. c, Velocities decompose into in-plane and out-of-plane tissue motions, here shown by a 2D pullback representation of the tangential tissue velocity (colored quiverplot) and the normal velocity, (red for inward velocity, blue for outward). d, We integrate tissue deformations over time in the tissue’s material frame of reference. Here, the gut is colored by the location of each tissue parcel in its intrinsic material coordinate system . Patches retain their original color as they move, stretch, and bend. e, Tracking individual cells typically involves laborious manual input and does not readily return tissue-scale deformation patterns. Cell identities are colored from an in silico dataset of cells on a coiling tube. f, Cartographic projections using previously-published methods fail for complex and dynamic shapes such as the folding midgut. (Left) Parameterization errors appear when using ImSAnE’s cylinderMeshWrapper on complex surfaces. (Right) Motion of cells in the pullback plane is large for adjacent timepoints. g, For the same in silico dataset as in (e), TubULAR maps the tissue to a series of images which change little over time. By tracking the motion in 2D, we read out tissue deformation across the full tube in 3D, here shown using the accumulated dilatational strain.
