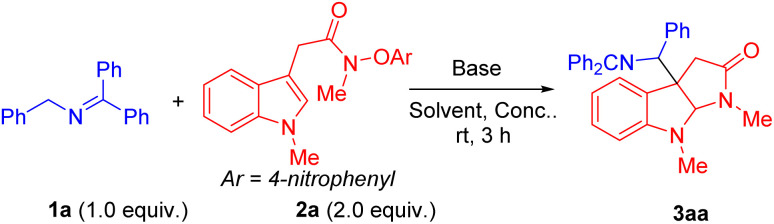
Optimization of coupling of ketimine 1a and amide 2aa,b.
| ||||
|---|---|---|---|---|
| Entry | Base (equiv.) | Solvent | Conc. [M] | Yield (%)b |
| 1 | LiOtBu (1.5) | DMSO | 0.2 | 56 (dr = 1 : 1) |
| 2 | NaOtBu (1.5) | DMSO | 0.2 | 61 (dr = 1 : 1) |
| 3 | KOtBu (1.5) | DMSO | 0.2 | 65 (dr = 1.3 : 1) |
| 4 | LiHMDS (1.5) | DMSO | 0.2 | 59 (dr = 1 : 1) |
| 5 | NaHMDS (1.5) | DMSO | 0.2 | 89 (86)c (dr = 1.2 : 1) |
| 6 | KHMDS (1.5) | DMSO | 0.2 | 65 (dr = 1 : 1) |
| 7 | NaHMDS (1.5) | THF | 0.2 | 0 |
| 8 | NaHMDS (1.5) | DMF | 0.2 | 0 |
| 9 | NaHMDS (1.5) | CPME | 0.2 | 0 |
| 10 | NaHMDS (1.5) | MTBE | 0.2 | 0 |
| 11 | NaHMDS (1.5) | MeCN | 0.2 | 0 |
| 12 | NaHMDS (1.5) | DMSO | 0.1 | 78 (dr = 1 : 1) |
| 13 | NaHMDS (1.5) | DMSO | 0.05 | 68 (dr = 1 : 1) |
| 14 | NaHMDS (2.0) | DMSO | 0.2 | 72 (dr = 1 : 1) |
| 15d | NaHMDS (1.5) | DMSO | 0.1 | 22 (dr = 1 : 1) |
| 16e | NaHMDS (1.5) | DMSO/THF = 1 : 1 | 0.1 | 8 (dr = 1 : 1) |
Reaction conditions: 1a (0.1 mmol, 1.0 equiv), 2a (0.2 mmol, 2.0 equiv), base, rt., 3 h.
Assay yield (AY) determined by 1H NMR spectroscopy of the crude reaction mixture using C2H2Cl4 as an internal standard.
Isolated yield after chromatographic purification.
60 °C.
0 °C.
