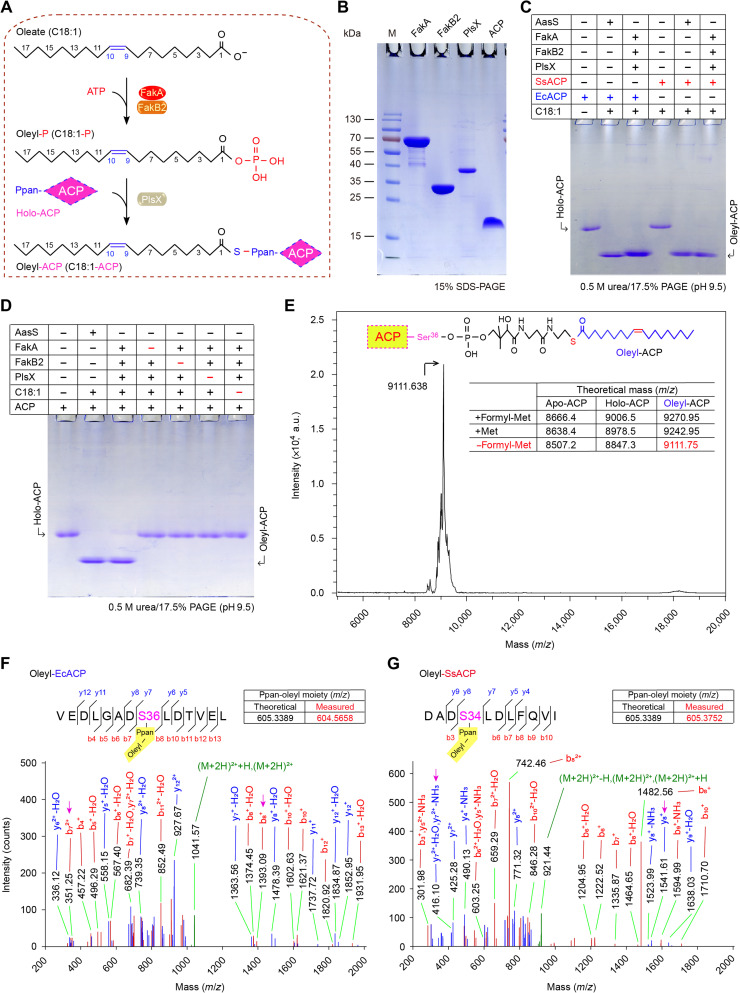
Fig. 2. Biochemical evidence that the Fak-PlsX system catalyzes the production of oleyl-ACP via the phosphorylated oleate intermediate.
(A) Scheme for catalytic actions by the Fak-PlsX reaction system. (B) Components used for the in vitro reconstitution of FakA/FakB2-PlsX enzymatic system. (C and D) In vitro reconstitution of FakA/FakB2-FlsX system to produce oleyl-ACP in the presence of SsACP (and/or EcACP). Conformationally sensitive 0.5 M urea/17.5% PAGE (pH 9.5) was used to track the oleyl-ACP product from FakA-FakB2 reaction system in the presence of PlsX. The symbol of plus (+) refers to addition of protein (and/or oleic acid), whereas minus (−) denotes no addition. (E) Use of matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) to measure molecular mass of the oleyl-ACP product. Molecular mass for the form of oleyl-ACP lacking its initial formyl-Met residue is measured to be 9111.638, close to its theoretical value of 9111.74. LC-MS identity for oleyl-EcACP (F) and oleyl-SsACP (G). Posttranslational modification with oleyl moiety occurs on the residue S36 of EcACP (F) and on S34 of SsACP (G). The actual mass of Ppan-oleyl moiety is separately measured by LC-MS to be 604.5658 and 605.3752, which matches its theoretical value of 605.3389. C18:1-P, oleyl-P; EcACP, E. coli ACP; SsACP, S. suis ACP; Met, methionine; formyl-Met, n-formyl-methionine.