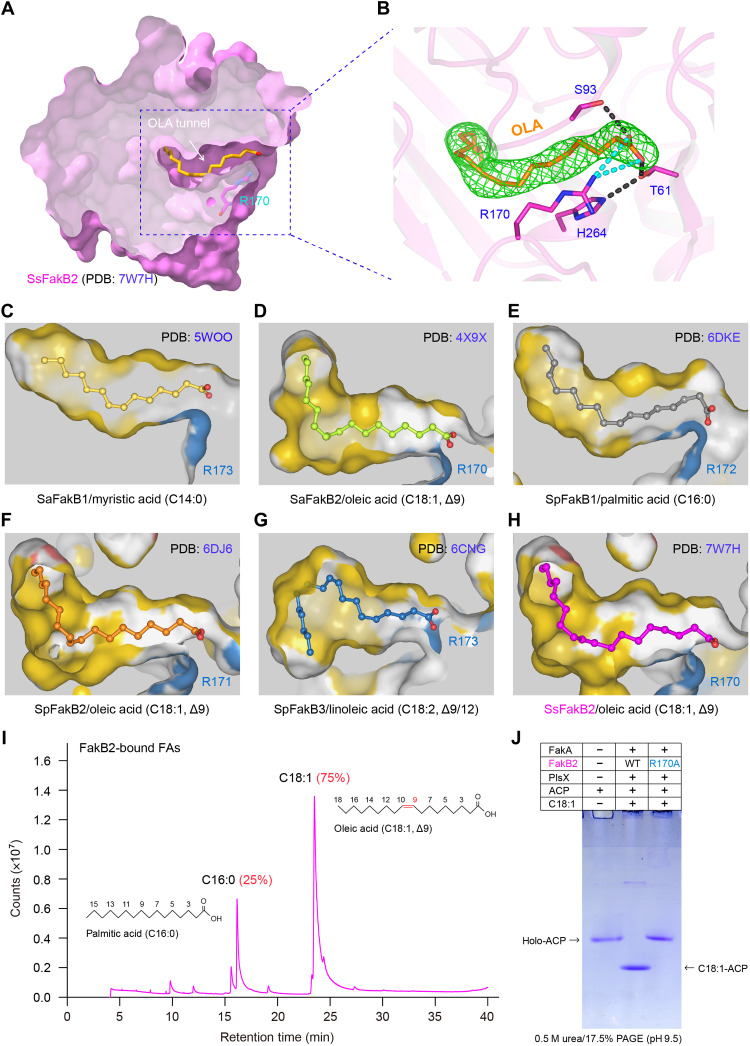
Fig. 7. Identification of an oleate-loading tunnel from SsFakB2 and its comparison with the counterparts in diverse FakB proteins.
(A) Sliced view of the substrate oleic acid (C18:1, Δ9)–loading tunnel of SsFakB2. (B) Fo-Fc omit map contoured at 2.0σ for the oleic acid molecule occupied within SsFakB2. (C) Sliced view of the myristic acid (C14:0)–loading cavity within SaFakB1 (PDB: 5WOO). (D) Structural insight into the oleic acid (C18:1, Δ9)–recognizing pocket of SaFakB2 (PDB: 4X9X). (E) Structural snapshot for the palmitic acid (C16:0)–loading tunnel from SpFakB1 protein (PDB: 6DKE). (F) Structural visualization for the oleic acid (C18:1, Δ9)–occupied funnel from SpFakB2 (PDB: 6DJ6). (G) Structural illustration for the linoleic acid (C18:1, Δ9/12)–binding tunnel within SpFakB3 (PDB: 6CNG). (H) Structural characterization of the oleate-loading pocket from SsFakB2 (PDB: 7W7H). (I) GC analysis suggested the presence of oleic acids predominantly bound in the FakB2 protein. (J) The FakB2 (R170A) substitution inactivates the ability of FakA/FakB2-PlsX in transferring exogenous oleic acids to ACP carrier.