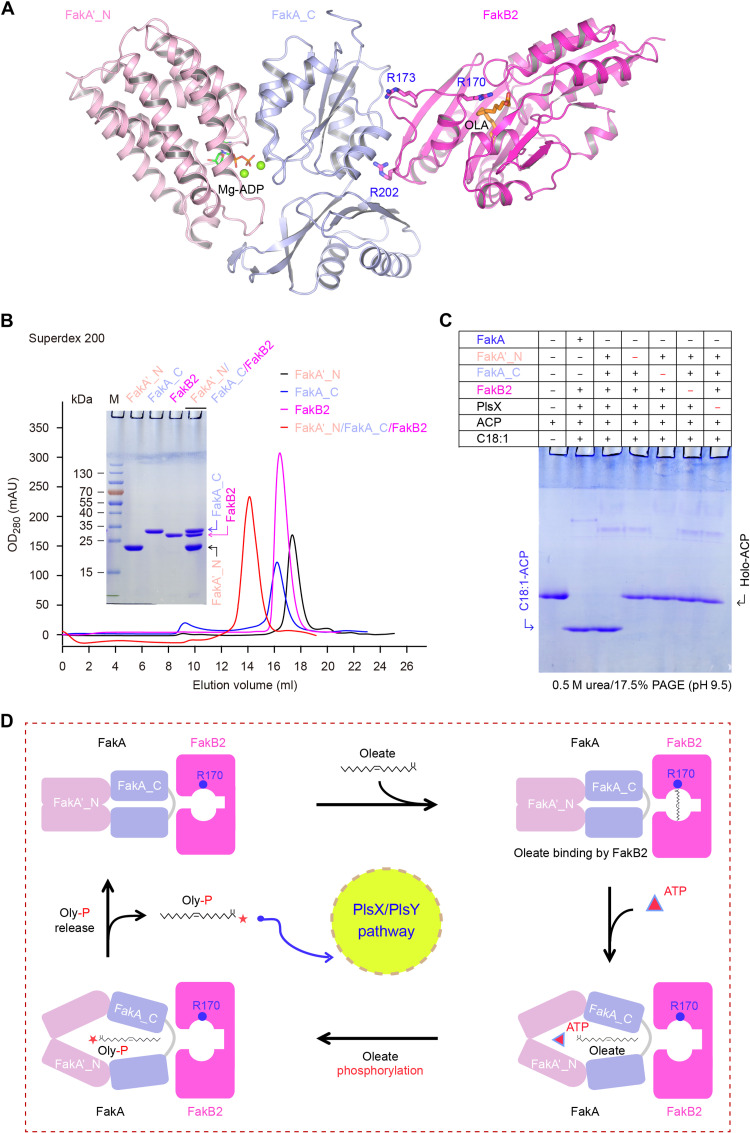
Fig. 8. A working model for oleate phosphorylation by Fak kinase system.
(A) Ribbon structure illustrating the interplay of FakA_C with both FakA′_N and FakB2. As for FakB2, three critical residues are highlighted, namely, (i) R170 required for fixation of oleate carboxyl group and (ii) R202 and R173 interacting with FakA_C domain. Mg-ADP is modeled into FakA_N′ based on the DhaL–Mg-ADP structure (PDB: 3PNL). (B) Gel filtration analysis of the ternary complex of FakA′_N, FakA_C, and FakB2. The inside gel denotes the protein identity. Size exclusion chromatography was conducted with a Superdex 200 increase column. (C) The in vitro reconstituted Fak system in which the full-length FakA is replaced with its two separated domains (FakA′_N plus FakA_C) retains the ability to produce oleyl-ACP. Given the fact that FakA_C binds to both FakA′_N domain and its partner FakB2, we attempted to test if the two domains (FakA′_N plus FakA_C) can partially replace the dimeric FakA in vitro. Relative to the full-length FakA (60 nM), each of the two domains (FakA′_N plus FakA_C) was supplemented at 500 to 600 nM in this in vitro reconstituted system. (D) Assignment of putative reaction steps to oleate phosphorylation by the FakA-FakB2 system.