Abstract
Background:
Patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA alterations have poor outcomes. MAGNITUDE found patients with homologous recombination repair gene alterations (HRR+), particularly BRCA1/2, benefit from first-line therapy with niraparib plus abiraterone acetate and prednisone (AAP). Here we report longer follow-up from the second prespecified interim analysis (IA2).
Patients and methods:
Patients with mCRPC were prospectively identified as HRR+ with/without BRCA1/2 alterations and randomized 1:1 to niraparib (200 mg orally) plus AAP (1000 mg/10 mg orally) or placebo plus AAP. At IA2, secondary endpoints (time to symptomatic progression, time to initiation of cytotoxic chemotherapy, overall survival [OS]) were assessed.
Results:
Overall, 212 HRR+ patients received niraparib plus AAP (BRCA1/2 subgroup, n=113). At IA2 with 24.8 months of median follow-up in the BRCA1/2 subgroup, niraparib plus AAP significantly prolonged radiographic progression-free survival (rPFS; blinded independent central review; median rPFS, 19.5 versus 10.9 months; hazard ratio [HR]=0.55 [95% confidence interval (CI) 0.39–0.78]; nominal P=0.0007) consistent with the first prespecified interim analysis. rPFS was also prolonged in the total HRR+ population (HR=0.76 [95% CI 0.60–0.97]; nominal P=0.0280; median follow-up, 26.8 months). Improvements in time to symptomatic progression and time to initiation of cytotoxic chemotherapy were observed with niraparib plus AAP. In the BRCA1/2 subgroup, the analysis of OS with niraparib plus AAP demonstrated an HR=0.88 (95% CI 0.58–1.34; nominal P = 0.5505); the prespecified inverse probability censoring weighting analysis of OS, accounting for imbalances in subsequent use of poly adenosine diphosphate-ribose polymerase inhibitors and other life-prolonging therapies demonstrated an HR=0.54 (95% CI 0.33–0.90; nominal P=0.0181). No new safety signals were observed.
Conclusions:
MAGNITUDE, enrolling the largest BRCA1/2 cohort in first-line mCRPC to date, demonstrated improved rPFS and other clinically relevant outcomes with niraparib plus AAP in patients with BRCA1/2-altered mCRPC, emphasizing the importance of identifying this molecular subset of patients.
Keywords: niraparib, abiraterone acetate, metastatic castration-resistant prostate cancer, BRCA, homologous recombination repair
INTRODUCTION
Metastatic castration-resistant prostate cancer (mCRPC) is a clinically heterogeneous disease associated with high mortality, despite recent improvements in therapeutic options.1–4 Up to 30% of patients with mCRPC harbor alterations in genes associated with DNA damage repair, including homologous recombination repair (HRR) genes, which are associated with poor clinical outcomes and earlier resistance to commonly used systemic therapies.5–10 Increasing evidence suggests that patients with mCRPC and BRCA1/2 alterations represent a distinct molecular subtype of mCRPC with a more aggressive clinical phenotype and worse prognosis.6,7,11–14 Therefore, the molecular profiling of tumors may be important to guide treatment decisions in patients with mCRPC and HRR-associated gene alterations, particularly for those involving BRCA. Practice guidelines generally recommend genetic testing of patients with mCRPC for mutations in DNA repair deficiency genes, particularly HRR-associated genes, to help tailor treatments to specific patient populations.15
Poly adenosine diphosphate-ribose polymerase (PARP) inhibitors have demonstrated significant activity in patients with prostate cancer and HRR mutations,9,16–19 with the greatest clinical benefit for BRCA1/2 mutation carriers.6,16,20–22 Niraparib, a highly selective PARP-1 and PARP-2 inhibitor approved for several indications, including ovarian, fallopian tube, and primary peritoneal cancers in select patients,23–27 is being studied in patients with mCRPC in the ongoing phase III MAGNITUDE trial (ClinicalTrials.gov: NCT03748641). In MAGNITUDE, of 423 HRR-positive (HRR+) patients enrolled, 225 (53.2%) were BRCA1/2-positive, making it the largest cohort of BRCA1/2-positive patients with mCRPC studied in the first-line setting to date.28 Furthermore, MAGNITUDE was designed to be representative of first-line patients with mCRPC seen in clinical practice by allowing patients to have recently received next-generation androgen receptor inhibitors for metastatic castration-sensitive prostate cancer (mCSPC) and nonmetastatic castration-resistant prostate cancer (CRPC), as well as permitting up to 4 months of abiraterone acetate with prednisone (AAP) for first-line mCRPC before enrollment to allow for time to perform genomic analyses and obtain results. The first interim analysis (IA1) of MAGNITUDE, with a median duration of follow-up in the HRR+ cohort of 18.6 months, demonstrated that niraparib plus AAP significantly improved the primary endpoint of radiographic progression-free survival (rPFS) in patients with mCRPC and HRR gene alterations. Of note, a preplanned futility analysis in patients with mCRPC without HRR gene alterations showed no benefit for the combination of niraparib plus AAP.28 Here, we report updated results from the second interim analysis (IA2) of the MAGNITUDE trial, with a focus on the preplanned subgroup analysis of patients with BRCA1/2 alterations.
PATIENTS AND METHODS
Study design and oversight
The methods of this ongoing randomized, double-blinded, placebo-controlled phase III trial have been previously published and will be discussed briefly herein.28 The study protocol and amendments were reviewed by an Independent Ethics Committee or Institutional Review Board, and all applicable regulatory requirements were followed. This study was conducted following the ethical principles outlined in the Declaration of Helsinki and is consistent with International Conference on Harmonisation and Good Clinical Practice guidelines. Patients or their legal representatives provided their written informed consent to participate in the study.
Patients
Eligible patients were aged ≥18 years, had mCRPC, and had not received prior therapy for mCRPC except up to 4 months of prior AAP and ongoing androgen deprivation therapy. Patients had to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients could have had systemic therapies for mCSPC or nonmetastatic CRPC, including androgen receptor-targeted therapy (e.g., apalutamide, darolutamide, or enzalutamide). Patients were prospectively screened for HRR gene alterations (ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, or PALB2) from blood and/or tumor tissue (archival or recently collected) samples. Assays used included FoundationOne tissue test (FoundationOne®CDx), Resolution Bioscience liquid test (ctDNA), AmoyDx® blood and tissue assays, or accredited local lab test results demonstrating a pathogenic germline or somatic alteration outlined in the study protocol (Supplementary Figure S1).
Study treatments
Patients were randomized 1:1 to receive either niraparib (200 mg) plus abiraterone acetate (1000 mg) and prednisone (10 mg) or placebo plus AAP, all administered daily. Study treatments were administered on an outpatient basis, with each treatment cycle defined as 28 days. Patients were stratified by past taxane-based chemotherapy exposure (yes versus no), past androgen receptor-targeted therapy exposure (prior novel anti-androgen therapy, such as enzalutamide, apalutamide, darolutamide versus no prior novel anti-androgen therapy), prior AAP use (yes versus no), and gene alteration (i.e., BRCA1 or BRCA2 versus all other HRR gene alterations).
Assessments and outcomes
The primary endpoint was rPFS as assessed by blinded independent central review and defined as the earlier of first progression on imaging (by bone scan, computed tomography, or magnetic resonance imaging) or death. Secondary endpoints included time to symptomatic progression, defined as the earliest time to any of the following: use of external beam radiation therapy for skeletal symptoms, the need for tumor-related orthopedic surgical intervention, other cancer-related procedures, cancer-related morbid events, or initiation of a new systemic anticancer therapy because of cancer pain; time to initiation of cytotoxic chemotherapy; and overall survival (OS). Other endpoints included time to prostate-specific antigen (PSA) progression, time to pain progression, time to pain interference, and overall health-related quality of life (HRQOL) using Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire total scores. Safety was assessed based on the occurrence of adverse events (AE), clinical laboratory test results, vital sign measurements, physical examination, and ECOG PS.
Statistical analysis
The statistical methods for the MAGNITUDE trial have been described previously.28 In brief, the primary endpoint of rPFS by blinded independent central review was powered for and tested using a 2-sided alpha level of 0.05 first in the BRCA1/2 subgroup (patients with BRCA1 and/or BRCA2 alterations). If statistical significance was reached, testing proceeded to rPFS in the HRR+ population. If rPFS in HRR+ showed statistical significance, then the secondary endpoints (time to symptomatic progression, time to initiation of cytotoxic chemotherapy, and OS) in HRR+ would be tested using a group sequential method with 2 interim analyses and a final analysis. At IA1, the primary endpoint of rPFS by blinded independent central review in the BRCA1/2 subgroup and HRR+ population was statistically significant; however, given that the secondary endpoints did not reach the conservative boundary (P = 0.0001; O’Brien-Fleming method) for statistical significance, IA2 was performed to reassess the impact of niraparib plus AAP on secondary endpoints. The prespecified IA2 was scheduled when approximately 170 OS events had occurred. Because the primary endpoint had already demonstrated statistical significance at IA1, no formal statistical testing for rPFS was performed at IA2, but formal testing was performed on the HRR+ population for the secondary endpoints of time to symptomatic progression, time to initiation of cytotoxic chemotherapy, and OS. P values were derived from a log-rank test stratified by past taxane-based chemotherapy exposure, prior AAP use, and gene alteration (for HRR+ population: BRCA1/BRCA2 versus all other HRRs); estimates of hazard ratios (HR) were calculated from the stratified proportional hazards model. A preplanned sensitivity analysis for OS was performed to adjust for the imbalance between the 2 treatment groups receiving subsequent PARP inhibitors and other life-prolonging therapies, by applying inverse probability censoring weighting (IPCW). The overall study-wise type I error rate remained adequately controlled at the 2-sided level of 0.05.
RESULTS
Patient characteristics
A total of 423 patients with ≥1 HRR alteration were enrolled, of whom 225 (53.2%) had alterations in BRCA1 or BRCA2 (Supplementary Figure S1). At the IA2 data cutoff (17 June 2022), HRR+ patients were on treatment for a median of 17.9 months and 15.2 months for the niraparib plus AAP and placebo plus AAP groups, respectively, with 74 (34.9%) and 57 (27.0%) patients, respectively, on treatment at the time of data cutoff. The baseline characteristics of the BRCA1/2 subgroup and the HRR+ population are presented in Table 1. As previously reported,29 poor prognostic factors, such as higher ECOG PS (1 versus 0) and the presence of visceral metastases, were more frequently reported in the niraparib plus AAP arm.
Table 1.
Baseline characteristics
| BRCA1/2 subgroup | HRR+ population | |||
|---|---|---|---|---|
| NIRA + AAP (n = 113) | PBO + AAP (n = 112) | NIRA + AAP (n = 212) | PBO + AAP (n = 211) | |
| Median age, (range) years | 67 (45–100) | 68 (43–88) | 69 (45–100) | 69 (43–88) |
| ECOG PS, n (%) 0 / 1 | 69 (61.1) / 44 (38.9) | 80 (71.4) / 32 (28.6) | 130 (61.3) / 82 (38.7) | 146 (69.2) / 65 (30.8) |
| Bone metastases, n (%) | 99 (87.6) | 93 (83.0) | 183 (86.3) | 170 (80.6) |
| Visceral metastases, n (%) | 26 (23.0) | 22 (19.6) | 51 (24.1) | 39 (18.5) |
| Liver | 10 (8.8) | 7 (6.3) | 18 (8.5) | 13 (6.2) |
| Lung | 12 (10.6) | 11 (9.8) | 27 (12.7) | 18 (8.5) |
| PSA at study entry (μg/l), median (range) | 18.7 (0.1–2225.8) | 14.1 (0.1–4400.0) | 21.4 (0–4826.5) | 17.4 (0.1–4400.0) |
| Prior taxane-based chemotherapy for nmCRPC/mCSPC, n (%) | 26 (23.0) | 29 (25.9) | 41 (19.3) | 44 (20.9) |
| Prior AR-targeted therapy for nmCRPC/mCSPC, n (%) | 6 (5.3) | 5 (4.5) | 8 (3.8) | 5 (2.4) |
| Prior AAP therapy for L1 mCRPC,a n (%) | 30 (26.5) | 29 (25.9) | 50 (23.6) | 48 (22.7) |
| Key laboratory values, median (range) | ||||
| Alkaline phosphatase enzyme, U/l | 111.0 (36.0–5234.0) | 97.0 (47.0–1892.0) | 106.0 (36.0–5234.0) | 100.0 (47.0–2651.0) |
| Hemoglobin, g/l | 128.0 (64.0–160.0) | 131.0 (75.0–161.0) | 129.0 (64.0–172.0) | 131.0 (75.0–161.0) |
| Lactate dehydrogenase enzyme, U/l | 204.0 (98.0–2959.0) | 197.0 (98.0–1530.0) | 194.0 (84.0–645.0) | 202.0 (131.0–758.0) |
AAP, abiraterone acetate with prednisone; AR, androgen receptor; ECOG PS, Eastern Cooperative Oncology Group performance status; HRR, homologous recombination repair; L1, first line; mCRPC, metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; NIRA, niraparib; nmCRPC, nonmetastatic castration-resistant prostate cancer; PBO, placebo; PSA, prostate-specific antigen.
Patients could have received up to 4 months of AAP before study entry in the mCRPC setting.
Efficacy results
At time of data cutoff in IA2, with 8.1 months of additional follow-up from the IA1 analysis, rPFS by blinded independent central review demonstrated a consistent and clinically meaningful treatment effect in the BRCA1/2 subgroup. The risk of progression or death was reduced by 45% in patients who received niraparib plus AAP (HR = 0.55 [95% confidence interval (CI) 0.39–0.78]; nominal P = 0.0007), lengthening the median rPFS by 8.6 months for patients who received niraparib plus AAP (19.5 months) compared with placebo plus AAP (10.9 months; Figure 1). A preplanned sensitivity analysis of the BRCA1/2 subgroup evaluating rPFS by investigator review also showed benefit for niraparib plus AAP, extending the median rPFS by 15.5 months (median rPFS of 29.3 months versus 13.8 months; HR = 0.46 [95% CI 0.32–0.67]; nominal P < 0.0001; Supplementary Figure S2A). In the preplanned multivariate analysis of rPFS adjusting for baseline disease characteristics, the benefit of receiving niraparib plus AAP was confirmed (HR = 0.50 [95% CI 0.35–0.71; Supplementary Table S1). Evaluation of rPFS by different baseline clinical and disease characteristics for the BRCA1/2 subgroup demonstrated consistent results in favor of niraparib plus AAP. Only the HR point estimates for patients with prior taxane-based chemotherapy and presence of visceral metastases were >0.9 (Supplementary Figure S3); however, the sample sizes of these subgroups were small (n = 53 and n = 48, respectively). Results for rPFS in the HRR+ population also demonstrated a clinically meaningful treatment effect favoring niraparib plus AAP (HR = 0.76 [95% CI 0.60–0.97]; nominal P = 0.0280) with median rPFS by blinded independent central review for niraparib plus AAP versus placebo plus AAP of 16.7 months versus 13.7 months (Supplementary Figure S2B). For rPFS by investigator review in the HRR+ population, median rPFS was 22.3 months for niraparib plus AAP and 13.9 months for placebo plus AAP (Supplementary Figure S2C).
Figure 1. Radiographic progression-free survival at IA2 by blinded independent central review in the BRCA1/2 subgroup.
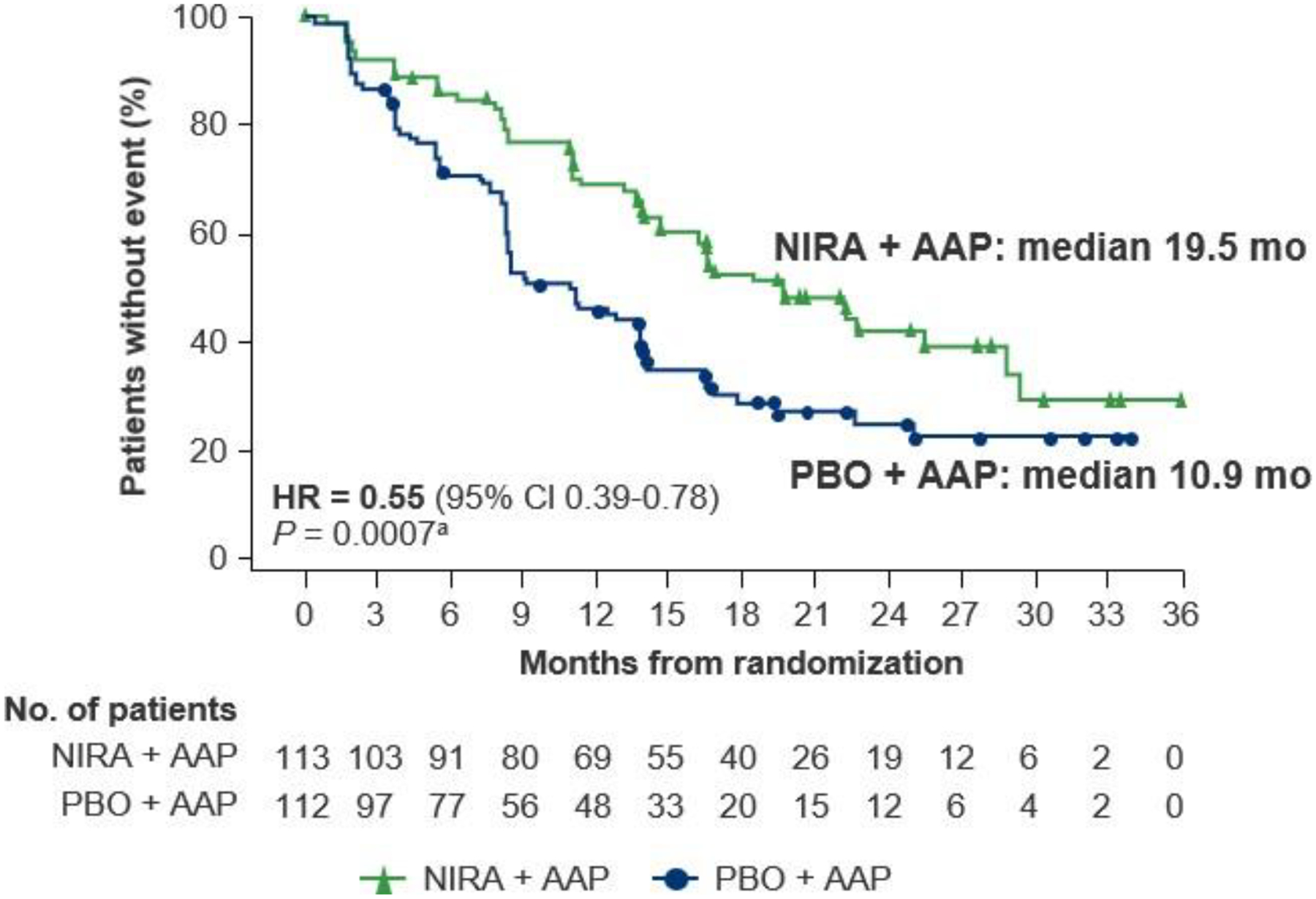
AAP, abiraterone acetate with prednisone; CI, confidence interval; HR, hazard ratio; IA2, second interim analysis; NIRA, niraparib; PBO, placebo. aNominal P value.
In the BRCA1/2 subgroup, an improvement in time to symptomatic progression was observed in patients who received niraparib plus AAP compared with placebo plus AAP (HR = 0.54 [95% CI 0.35–0.85]; nominal P = 0.0071; Figure 2). In the HRR+ population, a statistically significant and clinically meaningful prolongation in time to symptomatic progression was observed in patients treated with niraparib plus AAP compared with placebo plus AAP (HR = 0.60 [95% CI 0.42–0.84]; P = 0.0029; Supplementary Figure S4).
Figure 2. Time to symptomatic progression at IA2 in the BRCA1/2 subgroup.
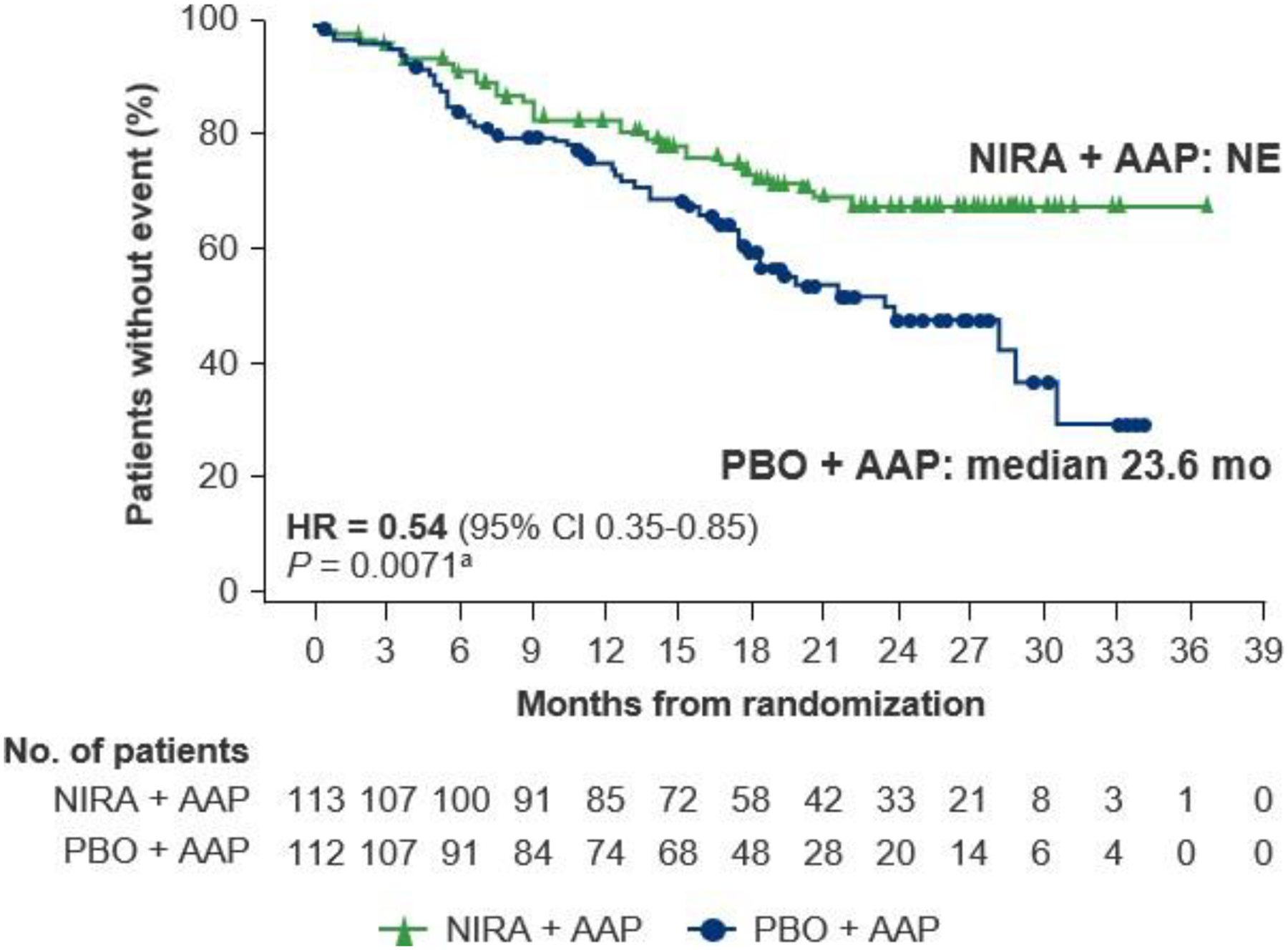
AAP, abiraterone acetate with prednisone; CI, confidence interval; HR, hazard ratio; IA2, second interim analysis; NE, not evaluable; NIRA, niraparib; PBO, placebo. aNominal P value.
In the BRCA1/2 subgroup, a clinically meaningful improvement in time to initiation of cytotoxic chemotherapy, supporting the 44% reduction in the risk of requiring chemotherapy, was observed in the niraparib plus AAP group compared with the placebo plus AAP group (HR = 0.56 [95% CI 0.35–0.90]; nominal P = 0.0152; Figure 3). In the HRR+ population, time to initiation of cytotoxic chemotherapy was prolonged in patients treated with niraparib plus AAP (HR = 0.67 [95% CI 0.47–0.94]; P = 0.0206; Supplementary Figure S5).
Figure 3. Time to initiation of cytotoxic chemotherapy at IA2 in the BRCA1/2 subgroup.
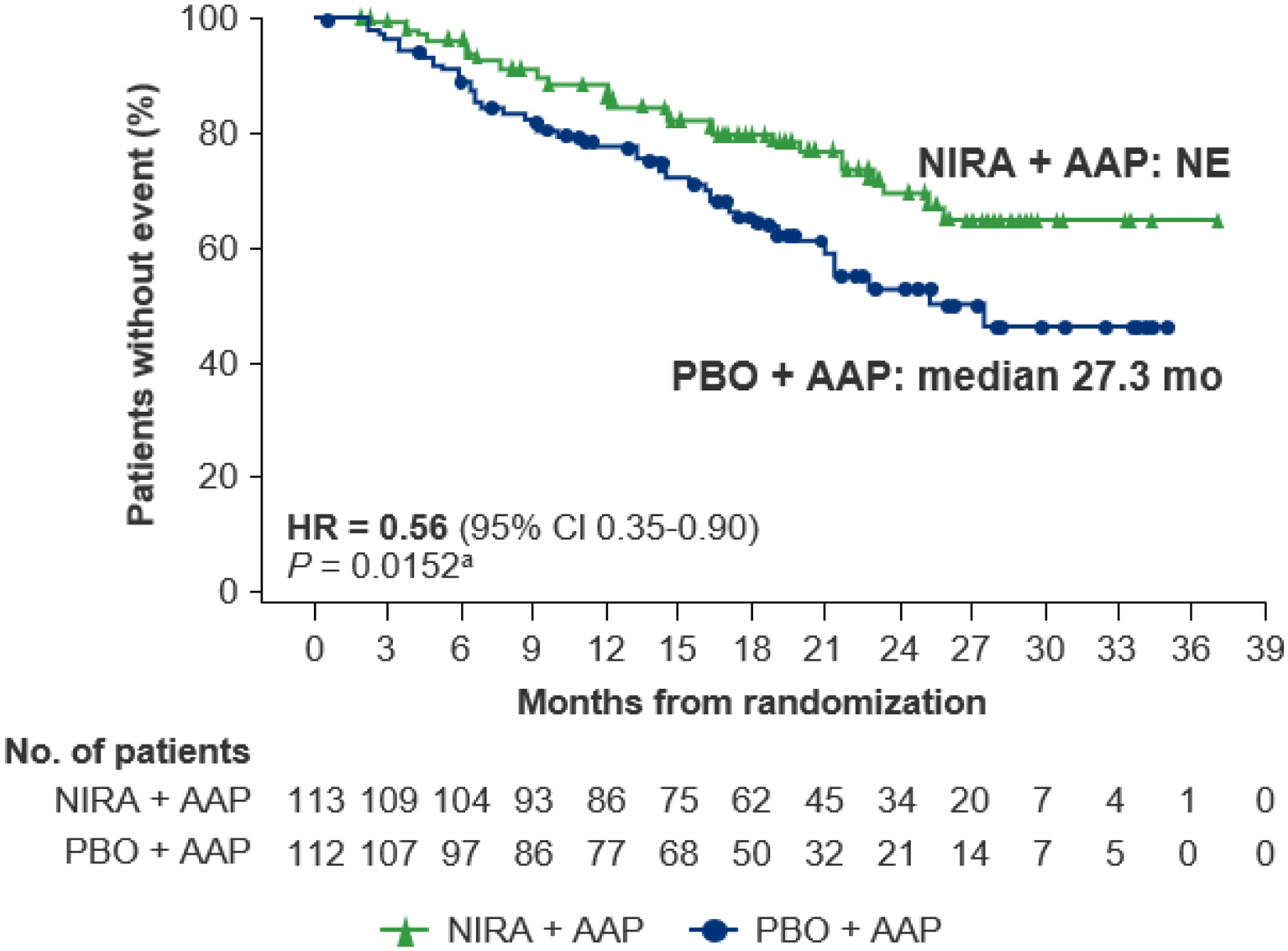
AAP, abiraterone acetate with prednisone; CI, confidence interval; HR, hazard ratio; IA2, second interim analysis; NE, not evaluable; NIRA, niraparib; PBO, placebo. aNominal P value.
With a median follow-up of 24.8 months (range, 0.5–36.8 months), in the BRCA1/2 subgroup at IA2, the HR for OS with niraparib plus AAP in the stratified analysis was 0.88 (95% CI 0.58–1.34; nominal P = 0.5505; Figure 4A). Substantially more patients with BRCA1/2 alterations in the placebo plus AAP arm received subsequent therapy (58.9%) versus the niraparib plus AAP group (31.0%); most notably, 22 (19.6%) patients in the placebo plus AAP arm received subsequent PARP inhibitor treatment versus 1 in the niraparib plus AAP arm (Supplementary Table S2). Accounting for imbalances in subsequent use of PARP inhibitors and other life-prolonging therapies, the prespecified IPCW analysis of OS showed a 46% reduction in the risk of death with niraparib plus AAP compared with placebo plus AAP in the BRCA1/2 subgroup (HR = 0.54 [95% CI 0.33–0.90]; nominal P = 0.0181; Figure 4B). The prespecified OS multivariate analysis accounting for important prognostic factors also showed longer OS with niraparib plus AAP in the BRCA1/2 subgroup (HR = 0.68 [95% CI 0.45–1.05]; nominal P = 0.0793; Supplementary Table S3). With a median follow-up of 26.8 months (range, 0.3–37.1) in the HRR+ population, 72.8% of deaths required for the final OS analysis had been observed. In the OS stratified analysis, HR was 1.01 (95% CI 0.75–1.36); P = 0.9480; Supplementary Figure 6A). After adjusting for baseline characteristics in a multivariate analysis (Supplementary Table S4) and adjusting for subsequent PARP inhibitors and other life-prolonging therapies in an IPCW analysis, OS improvement was observed in the HRR+ population (HR = 0.82 [95% CI 0.60–1.10]; nominal P = 0.1821, and HR = 0.70 [95% CI 0.49–0.99]; nominal P = 0.0414, respectively; Supplementary Table S4 and Supplementary Figure S6B). In the HRR+ population, 24 (11.4%) patients in the placebo plus AAP arm received subsequent PARP inhibitor treatment versus 1 in the niraparib plus AAP arm.
Figure 4. Overall survival at IA2 in the BRCA1/2 subgroup: (A) stratified analysis and (B) IPCW analysis.
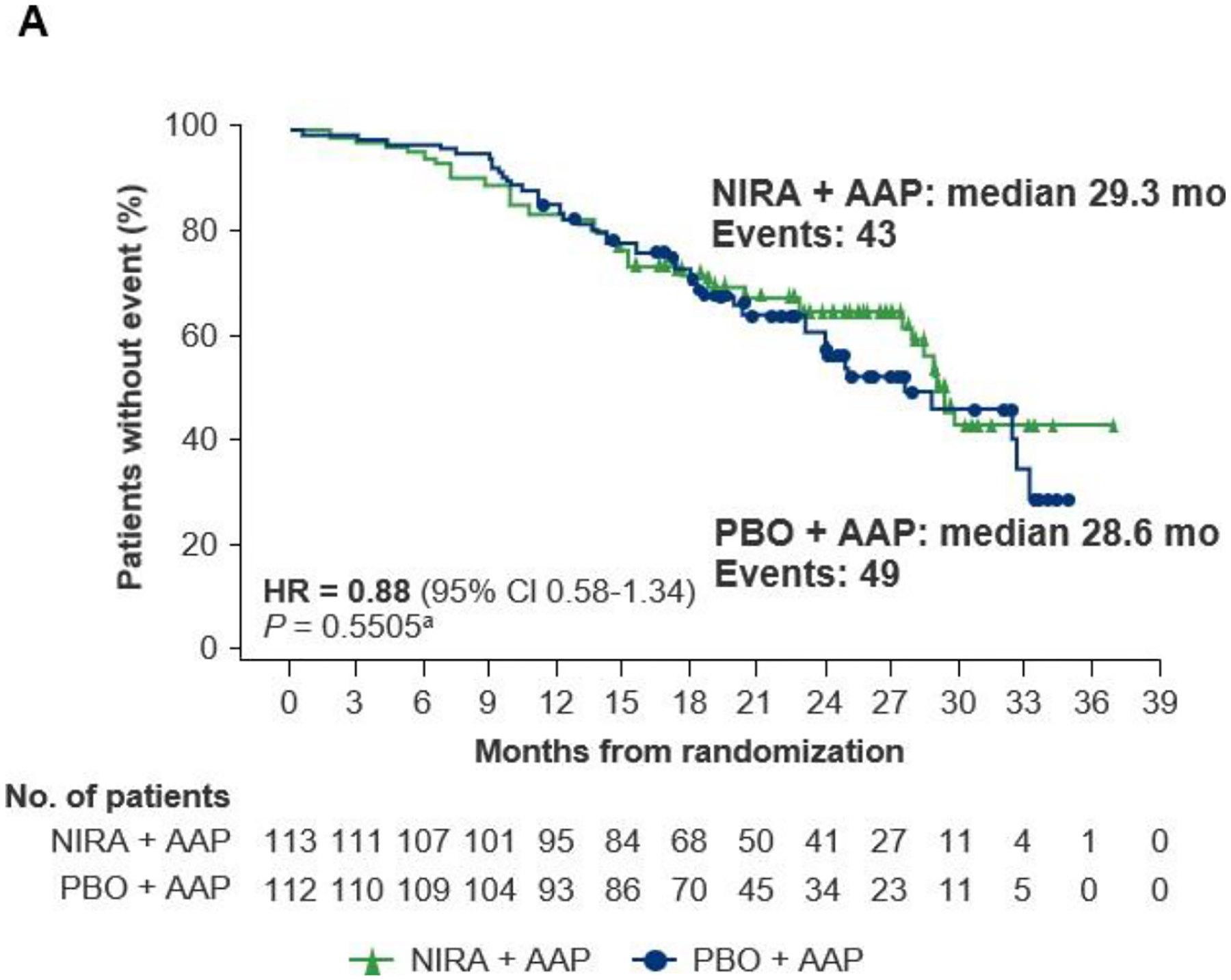
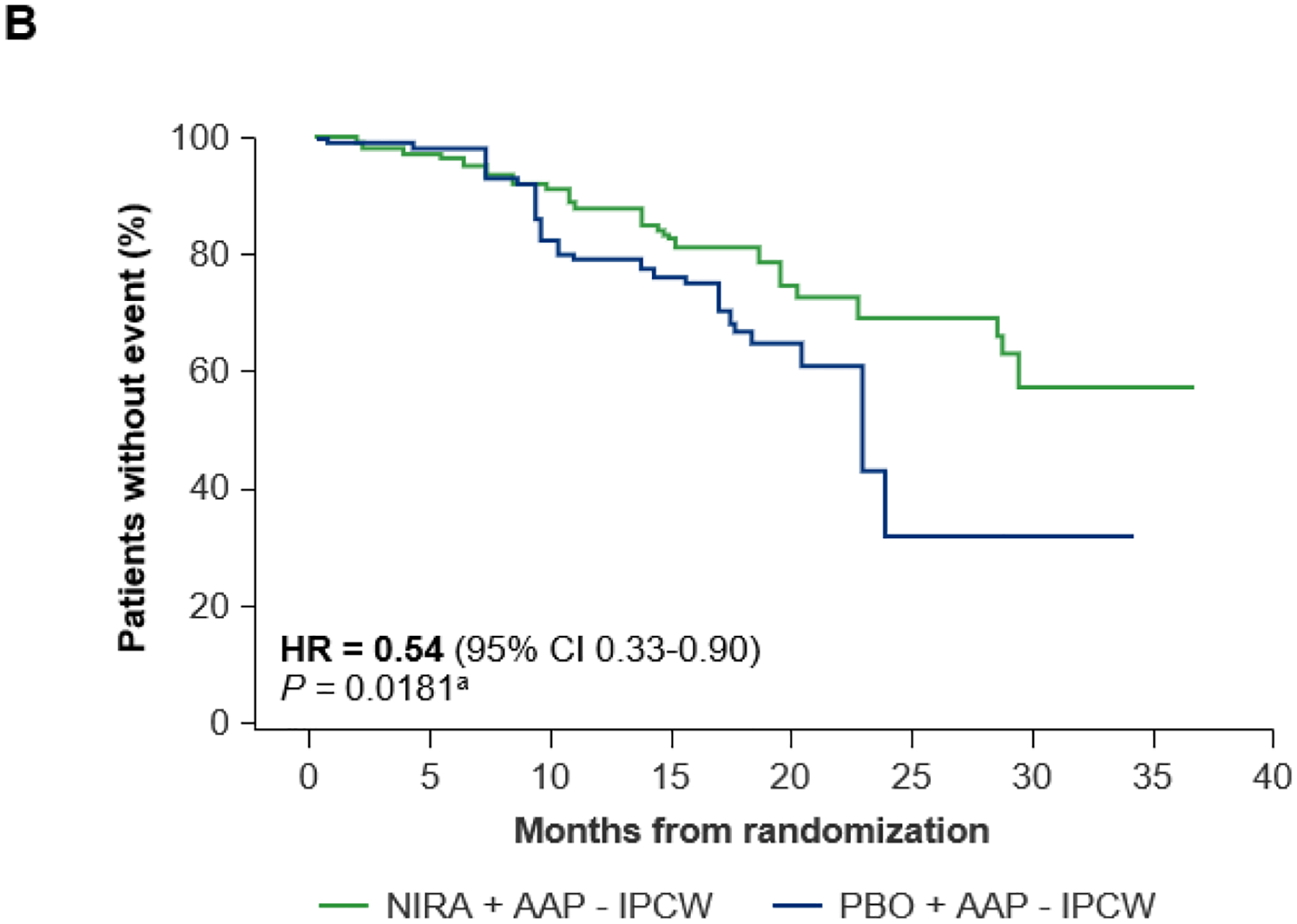
AAP, abiraterone acetate with prednisone; CI, confidence interval; HR, hazard ratio; IA2, second interim analysis; IPCW, inverse probability censoring weighting; NIRA, niraparib; PBO, placebo. aNominal P value.
In the BRCA1/2 subgroup, the median time to PSA progression doubled in the niraparib plus AAP group (18.4 months) versus the placebo plus AAP group (9.2 months), with a HR of 0.48 (95% CI 0.33–0.70); nominal P < 0.0001 (Supplementary Table S3). In this same subgroup, the objective response rate was 50.0% for niraparib plus AAP versus 31.3% for placebo plus AAP, yielding a relative risk for response of 1.60 (95% CI 0.98–2.62; nominal P = 0.053), and a relative risk for PSA response of 1.21 (95% CI 1.02–1.43; nominal P = 0.023).
To further define which gene alterations may identify patients who could derive greater benefit from treatment with niraparib plus AAP, sensitivity analyses were conducted for key efficacy endpoints, including rPFS, time to symptomatic progression, time to initiation of cytotoxic chemotherapy, and OS, in each individual gene alteration group and in functionally related subgroups (Supplementary Table S5). When patients with HRR-Fanconi Anemia pathway gene alterations (PALB2, BRIP1, FANCA) were analyzed together as a functionally related group, clinical benefit was demonstrated (point estimate for HR <1) across all primary and secondary endpoints for patients treated with niraparib plus AAP. Similar benefits across these same endpoints were observed for the functionally related group of patients with HRR-associated CHEK2 and HDAC2 gene alterations.
Patient-reported outcomes
In the BRCA1/2 subgroup, patients treated with niraparib plus AAP experienced a delay in time to worst pain intensity (HR = 0.70 [95% CI 0.44–1.12]; nominal P = 0.1338; Supplementary Figure S7A). Although the median time to pain interference was not reached for either treatment arm in the BRCA1/2 subgroup, at the 25th percentile, time to pain interference was 13.5 months with niraparib plus AAP and 12.9 months with placebo plus AAP (HR = 0.67 [95% CI 0.40–1.12]; nominal P = 0.1275; Supplementary Figure S7B). Overall HRQOL (FACT-P total score) in the BRCA1/2 subgroup was maintained during treatment in both the niraparib plus AAP and placebo plus AAP groups. Time to deterioration in FACT-P total scores was not different between treatment groups (median [95% CI] niraparib plus AAP = 5.5 months [2.9–7.5]; placebo plus AAP = 6.1 months [3.8–11.1]; HR = 1.07 [95% CI 0.76–1.50]; nominal P = 0.7144).
Safety
With a median exposure of 17.9 months in the niraparib plus AAP arm at IA2, the safety profile in the HRR+ population was consistent with that of IA1, with no new safety signals observed. AEs were experienced by 211 (99.5%) and 203 (96.2%) patients in the niraparib plus AAP and placebo plus AAP groups, respectively (Table 2). The most common (≥30%) AEs for niraparib plus AAP versus placebo plus AAP, regardless of causality, were anemia (50.0% versus 22.7%), hypertension (33.0% versus 22.3%), and constipation (33.0% versus 15.6%). Transfusion support for anemia was required by 27.4% of patients in the niraparib plus AAP group and by 5.2% of patients in the placebo plus AAP group, with 16.8% and 2.5%, respectively, receiving only 1 transfusion. Grade ≥3 AEs were observed in 153 (72.2%) patients in the niraparib plus AAP group and 104 (49.3%) patients in the placebo plus AAP group, of which the most common (≥10%) were anemia (30.2% versus 8.5%) and hypertension (15.6% versus 12.3%). The highest grade of hypertension observed was grade 3 (clinical stage 2) per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, no events of hypertensive crises or posterior reversible encephalopathy syndrome were observed, and no patients discontinued treatment due to hypertension. Observations were similar in the BRCA1/2 subgroup, with the exception of grade 3 hypertension, which occurred more frequently in the placebo plus AAP group (15.2%) versus the niraparib plus AAP group (13.3%).
Table 2.
TEAEs in the HRR+ population (occurring in >10% of patients)
| Event, n (%) | NIRA + AAP (n = 212) | PBO + AAP (n = 211) | ||||
|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Patients with ≥1 SAE | 93 (43.9) | 61 (28.9) | ||||
| Any TEAEs | 211 (99.5) | 121 (57.1) | 32 (15.1) | 203 (96.2) | 91 (43.1) | 13 (6.2) |
| Anemia | 106 (50.0) | 61 (28.8) | 3 (1.4) | 48 (22.7) | 18 (8.5) | 0 |
| Hypertension | 70 (33.0) | 33 (15.6) | 0 | 47 (22.3) | 26 (12.3) | 0 |
| Constipation | 70 (33.0) | 1 (0.5) | 0 | 33 (15.6) | 0 | 0 |
| Fatigue | 63 (29.7) | 8 (3.8) | 0 | 40 (19.0) | 11 (5.2) | 0 |
| Nausea | 52 (24.5) | 1 (0.5) | 0 | 31 (14.7) | 1 (0.5) | 0 |
| Thrombocytopenia | 49 (23.1) | 8 (3.8) | 8 (3.8) | 20 (9.5) | 5 (2.4) | 0 |
| Dyspnea | 38 (17.9) | 5 (2.4) | 0 | 14 (6.6) | 4 (1.9) | 0 |
| Back pain | 36 (17.0) | 6 (2.8) | 0 | 47 (22.3) | 2 (0.9) | 0 |
| Asthenia | 35 (16.5) | 2 (0.9) | 1 (0.5) | 21 (10.0) | 1 (0.5) | 0 |
| Decreased appetite | 33 (15.6) | 2 (0.9) | 0 | 15 (7.1) | 1 (0.5) | 0 |
| Arthralgia | 32 (15.1) | 1 (0.5) | 0 | 23 (10.9) | 2 (0.9) | 0 |
| Neutropenia | 32 (15.1) | 11 (5.2) | 3 (1.4) | 15 (7.1) | 4 (1.9) | 1 (0.5) |
| Vomiting | 31 (14.6) | 2 (0.9) | 0 | 16 (7.6) | 2 (0.9) | 0 |
| Hypokalemia | 29 (13.7) | 7 (3.3) | 1 (0.5) | 21 (10.0) | 7 (3.3) | 0 |
| Dizziness | 27 (12.7) | 1 (0.5) | 0 | 13 (6.2) | 0 | 0 |
| Hyperglycemia | 25 (11.8) | 6 (2.8) | 1 (0.5) | 18 (8.5) | 2 (0.9) | 0 |
| Insomnia | 24 (11.3) | 0 | 0 | 8 (3.8) | 0 | 0 |
| Bone pain | 23 (10.8) | 4 (1.9) | 0 | 24 (11.4) | 1 (0.5) | 0 |
| Blood alkaline phosphatase increased | 23 (10.8) | 10 (4.7) | 2 (0.9) | 16 (7.6) | 5 (2.4) | 0 |
| Leukopenia | 23 (10.8) | 4 (1.9) | 0 | 5 (2.4) | 1 (0.5) | 0 |
| Urinary tract infection | 22 (10.4) | 7 (3.3) | 0 | 18 (8.5) | 4 (1.9) | 0 |
| Weight decreased | 22 (10.4) | 3 (1.4) | 0 | 7 (3.3) | 1 (0.5) | 0 |
| Lymphopenia | 22 (10.4) | 8 (3.8) | 1 (0.5) | 4 (1.9) | 1 (0.5) | 1 (0.5) |
| Fall | 16 (7.5) | 2 (0.9) | 0 | 29 (13.7) | 6 (2.8) | 0 |
| Alanine aminotransferase increased | 11 (5.2) | 0 | 0 | 22 (10.4) | 10 (4.7) | 0 |
AAP, abiraterone acetate with prednisone; HRR, homologous recombination repair; NIRA, niraparib; PBO, placebo; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
In the niraparib plus AAP and placebo plus AAP groups, treatment-related AEs occurred in 165 (77.8%) and 121 (57.3%) patients, respectively, and were consistent with the known safety profiles of the individual drugs. Serious AEs were reported in 93 (43.9%) and 61 (28.9%) patients in the niraparib plus AAP and placebo plus AAP groups, respectively (Table 2). Pulmonary embolism occurred in 4 (1.9%) patients treated with niraparib plus AAP compared with 2 (0.9%) patients treated with placebo plus AAP. No cases of myelodysplastic syndrome or acute myeloid leukemia occurred in the niraparib plus AAP group versus 1 (0.5%) in the placebo plus AAP group; no cases of posterior reversible encephalopathy syndrome were observed in either group. Treatment-emergent AEs leading to dose interruption, dose reduction, or discontinuation of niraparib occurred in 49.1%, 20.3%, and 15.1% of patients in the niraparib plus AAP group, respectively, compared with 27.5%, 3.8%, and 5.7% of patients in the placebo plus AAP group, respectively. Anemia was the most common cause of dose interruption or dose reduction of niraparib, followed by thrombocytopenia and neutropenia.
Cumulatively through IA2, 29 (13.7%) and 23 (10.9%) patients died in the niraparib plus AAP and placebo plus AAP groups, respectively, while on treatment or within 30 days of the last dose of study treatment. Deaths due to AEs occurred in 19 (9.0%) and 9 (4.3%) patients in the niraparib plus AAP and placebo plus AAP groups, respectively, of which the most common AE leading to death was COVID-19 (4.7% and 0.9%, respectively). Since study initiation, 1 death in each group was categorized as treatment related (niraparib plus AAP: pneumonia; placebo plus AAP: acute myocardial infarction).
DISCUSSION
The results from IA2 of MAGNITUDE confirm that after a median follow-up of 24.8 months in patients with BRCA1/2 alterations, the combination of niraparib plus AAP continued to improve rPFS, demonstrating a 45% reduction in the risk of radiographic progression or death, which corresponded to an extension of the median rPFS by 8.6 months over placebo plus AAP, yielding a median rPFS of >1.5 years. The clinical relevance of the benefit in rPFS was supported by delays in time to symptomatic progression and time to initiation of cytotoxic chemotherapy for the BRCA1/2 subgroup and the total HRR+ population treated with niraparib plus AAP versus placebo plus AAP. Furthermore, while OS data are still maturing and a final analysis of the MAGNITUDE trial is planned, there was a nonstatistically significant improvement in OS observed in the stratified analysis for the BRCA1/2 subgroup, with a more robust effect observed in the analysis that accounted for imbalances in baseline characteristics. In addition, a prespecified IPCW analysis30,31 was conducted to account for the imbalances in subsequent receipt of PARP inhibitors (niraparib plus AAP: 0.9%; placebo plus AAP: 16.1%) and other life-prolonging therapies, including chemotherapy (niraparib plus AAP: 24.8%; placebo plus AAP: 39.3%). This IPCW analysis showed a 46% reduction in the risk of death with niraparib plus AAP compared with placebo plus AAP in the BRCA1/2 subgroup. This emphasizes the importance of subsequent therapy on OS in this patient population. While some other HRR-associated genes demonstrated benefit, the most pronounced benefit from niraparib plus AAP was in patients with BRCA1/2 alterations.32 This is consistent with other studies evaluating PARP inhibitors in patients with mCRPC and reflects the critical role of BRCA1/2 in the DNA damage repair response.33–35
The safety profile of niraparib plus AAP at IA2 was consistent with previous reports35 and the known safety profile of the individual agents, with no new safety signals identified. Anemia was the most common grade ≥3 AE, which aligns with observations from a meta-analysis of 6 trials encompassing 752 patients with mCRPC treated with olaparib, rucaparib, talazoparib, or niraparib.36 Further, the pooled incidence of treatment-related dose reduction was 26.9% and the incidence of treatment discontinuation due to AEs was 14.1%,36 which aligns with the findings of IA2 for MAGNITUDE. Overall, niraparib plus AAP was tolerable, and AEs were generally manageable with dose modifications and supportive care. There were no cases of hypertensive crisis or myelodysplastic syndrome and there was no apparent increase in thromboembolic events in the niraparib plus AAP arm. Supporting tolerability of the combination therapy, patients in the BRCA1/2 subgroup treated with niraparib plus AAP also maintained their HRQOL, as demonstrated by patient-reported overall quality of life scores.
The median rPFS of approximately 10 months for placebo plus AAP observed in this and other studies reflects the poor outcomes in patients with mCRPC and BRCA1/2 alterations when treated with standard-of-care therapies such as AAP alone, relative to the historically observed rPFS of 16.5 months in an unselected population treated with AAP alone.37–39 The clinically meaningful benefits of delay in chemotherapy and time to increased pain observed for the BRCA1/2 subgroup that received niraparib plus AAP as first-line therapy in this study support the use of this combination, particularly when considering the disease characteristics and poor prognosis of this population.
The study had some limitations. While many of the demographic factors and baseline characteristics of both the BRCA1/2 subgroup and total HRR+ population were balanced across the 2 treatment arms, several key baseline factors known to be prognostic for survival favored the placebo group.40 For example, more patients who received niraparib plus AAP versus placebo plus AAP had an ECOG PS of 1 (versus 0) and had bone and visceral metastases at baseline. Despite these differences, an improvement in OS was observed in the BRCA1/2 subgroup, which is notable given that more patients in the placebo arm received subsequent therapy, including 19.6% who received a PARP inhibitor. In addition, the germline versus somatic nature of the BRCA1/2 alterations was not determined in this study. While most studies of PARP inhibitors have not made this differentiation, the phase II TRITON2 study of rucaparib in patients who had previously progressed on 1 to 2 lines of androgen receptor–directed therapy did not demonstrate differences in PSA response (defined as the proportion of patients achieving a reduction of 50%) in those with germline versus somatic BRCA1/2 mutations.33,41 Also, a meta-analysis of patients with solid tumors showed no differences between those with germline versus somatic mutations, albeit the number of studies on prostate cancer was small.42
MAGNITUDE placed no restrictions on the extent of metastatic disease (unlimited bone and visceral metastases and asymptomatic brain metastases were permitted), allowed up to 4 months of prior AAP in the first-line mCRPC setting, and allowed prior taxane and androgen receptor systemic therapy for mCSPC. Among the 9 gene alterations permitted for enrollment, only BRCA1/2 alterations were powered to detect significant benefit of treatment for the endpoint of rPFS. Among the remaining 7 genes, benefit in rPFS and ≥1 secondary endpoint was demonstrated in BRIP1, CHEK2, FANCA, HDAC2, and PALB2 at IA132 and confirmed in IA2 (Supplementary Table S5); however, sample sizes remain small, and further study is required to define the benefit of combination therapy in patients with non–BRCA-altered mCRPC.
MAGNITUDE has prospectively enrolled the largest population of BRCA1/2-positive patients with mCRPC (n = 225) for first-line therapy to date and has demonstrated a clear positive risk-benefit in this difficult-to-treat population. These findings add to a growing body of evidence that supports the clinical benefit of PARP inhibitors, including niraparib, as monotherapy in HRR-altered mCRPC after progression on androgen receptor-targeted therapy and taxanes, especially for patients with BRCA1/2 alterations.33–35 The PARP inhibitors olaparib and talazoparib have shown efficacy in combination with AAP and enzalutamide, respectively, in studies of patients with mCRPC that were not preselected to have an HRR gene alteration (“all-comers” populations), but similarly to niraparib, the greatest benefits have been among patients with ≥1 alteration in BRCA1/2.20,43,44 Furthermore, the CAPTURE study of patients with mCRPC found that patients with BRCA1/2 alterations had shorter rPFS and OS compared with those who did not have BRCA1/2 alterations when treated with a first-line standard of care therapy (either a novel hormone therapy or a taxane-based regimen).45
In conclusion, the MAGNITUDE IA2 results support the treatment regimen of niraparib plus AAP in patients with BRCA1/2-altered mCRPC, with demonstration of continued improvements in rPFS, time to symptomatic progression, and time to initiation of cytotoxic chemotherapy. Thus, these results reinforce the need for genomic testing for patients with mCRPC in the first-line setting to identify those patients who would potentially derive optimal benefit in response to treatment with PARP inhibitors, such as niraparib.
Supplementary Material
Highlights.
Niraparib + AAP reduced risk of radiographic progression/death by 45% in BRCA1/2-altered mCRPC (median follow-up, 24.8 mo)
Niraparib + AAP improved secondary endpoints and patient-reported outcomes in the BRCA1/2 subgroup
Adverse events of niraparib + AAP were tolerable, manageable, and consistent with previous reports; no new safety signals
MAGNITUDE second interim analysis continues to support niraparib + AAP for mCRPC and HRR alterations, especially BRCA1/2
MAGNITUDE supports genomic testing for BRCA1/2 alterations in mCRPC due to poor outcomes and emerging treatment options
ACKNOWLEDGMENTS
We thank the patients who participated in this study, along with their families and caregivers, and the study teams who were involved at each participating institution. Medical writing support was provided by Emanuela Marcantoni, PhD, Meredith Rogers, MS, CMPP, and Jessica Deckman, PhD, CMPP, of the Lockwood Group (Stamford, CT, USA), which was provided in accordance with Good Publication Practice (GPP 2022) guidelines and funded by Janssen Research & Development, LLC.
FUNDING
This work was supported by Janssen Research & Development, LLC. No grants were obtained for the conduct of this work.
DISCLOSURE
KNC received consulting fees from Amgen, Astellas, AstraZeneca, Bayer, Janssen, Merck, Pfizer, POINT Biopharma, and Roche; and grants (to institution) from Amgen, AstraZeneca, Bayer, ESSA, Janssen, Merck, POINT Biopharma, and Roche. SS received honoraria from AstraZeneca, Bristol Myers Squibb, Janssen, Merck, and Novartis (DSM Committee Chair); and grants from AstraZeneca, Genentech, Merck, Novartis, and Pfizer. MRS received consulting or advisory fees from Amgen, Astellas, Bayer, Janssen, Eli Lilly, Novartis, and Pfizer; research funding from Bayer, ESSA, Janssen, Eli Lilly, and ORIC Pharmaceuticals; and travel, accommodations, and expense reimbursements from Amgen, Bayer, Janssen, and Eli Lilly. GA received honoraria from Astellas and Janssen; consulting or advisory fees from Abbott Laboratories, Astellas, AstraZeneca, Bayer, ESSA, Ferring, Janssen, Medivation, Millennium Pharmaceuticals, Novartis, Pfizer, Ventana Medical Systems, and Veridex; speakers’ bureau fees from Astellas, AstraZeneca, Ferring, Ipsen, Janssen, Sanofi, Takeda, and Ventana Medical Systems; research funding from Arno Therapeutics, Innocrin Pharma, and Janssen; has patents or other intellectual property or received royalties for abiraterone acetate; received travel, accommodations, or expense reimbursements from Abbott Laboratories, Astellas, Bayer, ESSA, Ferring, Janssen, Medivation, Pfizer, and Ventana Medical Systems; and is affiliated with the Institute of Cancer Research. MS received honoraria and consulting or advisory role fees from AstraZeneca, Astellas, Amgen, Ipsen, Johnson & Johnson, Merck, and Pfizer; grants (to institution) from Johnson & Johnson and Merck; and travel, accommodations, and expense reimbursements from Cipla, Ipsen, and Pfizer. DO received honoraria from Astellas, Bayer, and Janssen; consulting or advisory role fees from AstraZeneca, Bayer, Clovis Oncology, Daiichi Sankyo, Janssen, and Merck; research funding from Astellas, AstraZeneca, Bayer, Genentech/Roche, Janssen, Medivation, Merck, and Pfizer; and travel, accommodations, or expense reimbursements from Astellas, AstraZeneca, Bayer, Ipsen, Janssen, and Roche. EC received honoraria from Astellas, AstraZeneca, Bayer, Janssen, Merck, Pfizer, and Telix Pharmaceuticals; consulting or advisory fees from Astellas, AstraZeneca, Bayer, Daiichi Sankyo, Janssen, Merck, and Pfizer; grants (to institution) from AstraZeneca, Bayer, and Janssen; and travel, accommodations, or expense reimbursements from AstraZeneca, Bayer, Janssen, and Pfizer. GR received consulting or advisory role fees from Astellas, AstraZeneca (to institution), Bayer, Gilead (to institution), Janssen, Ipsen, and Pfizer; research funding from Bayer (to institution); honoraria for lectures/speakers’ bureau fees from AstraZeneca (to institution), Bayer, and Janssen; and travel, accommodations, or expense reimbursements from AstraZeneca, Bayer, and Janssen. ASG received honoraria for lectures/speakers’ bureau fees from Adium Pharma, Astellas, AstraZeneca, Bayer, and Janssen; and travel, accommodations, and expense reimbursements from Astellas and Janssen. EJS has stock or other ownership of Fortis and Teon Therapeutics; received honoraria from Janssen and Johnson & Johnson; and received consulting or advisory role fees from Fortis and Janssen. HG received consulting or advisory fees from Astellas, AstraZeneca, Ipsen, Janssen, Merck, Merck-Serono, and Pfizer; honoraria for lectures/speakers’ bureau fees from Ipsen and Merck; and received travel, accommodations, and expense reimbursements from AstraZeneca. WJ reports nothing to disclose. EE received consulting or advisory fees, speakers’ bureau fees, and research funding from Astellas, AstraZeneca, Merck, Myovant Sciences, Novartis, Pfizer, and Sanofi. GEM, SD, DW, BD, KU, AdC, PF, and WK are employed by Janssen Research & Development, LLC.
Footnotes
Meeting presentation: Presented in part at the American Society of Clinical Oncology Genitourinary (ASCO-GU) Cancers Symposium; February 16–18, 2023; San Francisco, CA, USA.
REFERENCES
- 1.Bieńkowski M, Tomasik B, Braun M, et al. PARP inhibitors for metastatic castration-resistant prostate cancer: biological rationale and current evidence. Cancer Treat Rev. 2022;104:102359. [DOI] [PubMed] [Google Scholar]
- 2.Henríquez I, Roach M 3rd, Morgan TM, et al. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines. 2021;9(9):1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DK, Chiu PK, Ng CF, et al. Novel strategies for treating castration-resistant prostate cancer. Biomedicines. 2021;9(4):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nava Rodrigues D, Casiraghi N, Romanel A, et al. RB1 heterogeneity in advanced metastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25(2):687–697. [DOI] [PubMed] [Google Scholar]
- 5.Abida W, Armenia J, Gopalan A, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017:PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(6):490–503. [DOI] [PubMed] [Google Scholar]
- 7.Cui M, Gao XS, Gu X, et al. BRCA2 mutations should be screened early and routinely as markers of poor prognosis: evidence from 8,988 patients with prostate cancer. Oncotarget. 2017;8(25):40222–40232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaram A, Wingate A, Wetterskog D, et al. Plasma tumor gene conversions after one cycle abiraterone acetate for metastatic castration-resistant prostate cancer: a biomarker analysis of a multicenter international trial. Ann Oncol. 2021;32(6):726–735. [DOI] [PubMed] [Google Scholar]
- 9.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner E, Herberts C, Fu S, et al. BRCA2, ATM, and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clin Cancer Res. 2021;27(6):1650–1662. [DOI] [PubMed] [Google Scholar]
- 11.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh M, Alkhushaym N, Fallatah S, et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: a meta-analysis. Prostate. 2019;79(8):880–895. [DOI] [PubMed] [Google Scholar]
- 14.Fazekas T, Széles AD, Teutsch B, et al. Therapeutic sensitivity to standard treatments in BRCA positive metastatic castration-resistant prostate cancer patients—a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. Published online December 12, 2022. doi: 10.1038/s41391-022-00626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatson EL, Chau CH, Price DK, et al. PARP inhibitors on the move in prostate cancer: spotlight on niraparib & update on PARP inhibitor combination trials. Am J Clin Exp Urol. 2022;10(4):252–257. [PMC free article] [PubMed] [Google Scholar]
- 19.Congregado B, Rivero I, Osmán I, et al. PARP inhibitors: a new horizon for patients with prostate cancer. Biomedicines. 2022;10(6):1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 21.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou E, Smith MR, Sandhu S, et al. Niraparib with abiraterone acetate and prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of MAGNITUDE. Poster presented at: American Society of Clinical Oncology Genitourinary (ASCO-GU) Cancers Symposium; February 16–18, 2023; San Francisco, CA. Poster 170. [Google Scholar]
- 23.GlaxoSmithKline. European Commission approves Zejula (niraparib) as first-line monotherapy maintenance treatment in advanced ovarian cancer. 2020. Available at https://www.gsk.com/en-gb/media/press-releases/european-commission-approves-zejula-niraparib-as-first-line-monotherapy-maintenance-treatment-in-advanced-ovarian-cancer/. Accessed March 21, 2023.
- 24.GlaxoSmithKline. ZEJULA (niraparib) prescribing information. 2022. Available at https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Zejula_Capsules/pdf/ZEJULA-CAPSULES-PI-PIL.PDF. Accessed March 21, 2023.
- 25.Canada’s Drug and Health Technology Agency. Niraparib (Zejula) for first line ovarian cancer - details. 2021. Available at https://www.cadth.ca/niraparib-zejula-first-line-ovarian-cancer-details. Accessed June 29, 2022.
- 26.Rosa K Niraparib approved in China for frontline maintenance in ovarian cancer. 2020. Available at https://www.onclive.com/view/niraparib-approved-in-china-for-frontline-maintenance-in-ovarian-cancer. Accessed June 29, 2022.
- 27.Washington CR, Moore KN. PARP inhibitors in the treatment of ovarian cancer: a review. Curr Opin Obstet Gynecol. 2021;33(1):1–6. [DOI] [PubMed] [Google Scholar]
- 28.Chi KN, Rathkopf D, Smith MR, et al. ; on behalf of the MAGNITUDE Principal Investigators. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023:JCO2201649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(6 suppl):12.34752147 [Google Scholar]
- 30.Latimer NR, Abrams KR. NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching. 2014. Available at https://www.fda.gov/media/167627/download. Accessed May 24, 2023. [PubMed]
- 31.European Medicines Agency. Question and answer on adjustment for cross-over in estimating effects in oncology trials. 2018. Available at https://www.ema.europa.eu/en/documents/scientific-guideline/question-answer-adjustment-cross-over-estimating-effects-oncology-trials_en.pdf. Accessed May 24, 2023.
- 32.Sandhu S, Attard G, Olmos D, et al. Gene-by-gene analysis in the MAGNITUDE study of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40(16 suppl):5020. [Google Scholar]
- 33.Abida W, Patnaik A, Campbell D, et al. ; on behalf of the TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250–1264. [DOI] [PubMed] [Google Scholar]
- 35.Smith MR, Scher HI, Sandhu S, et al. ; on behalf of the GALAHAD investigators. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2022;23(3):362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo A, Mollica V, Merler S, et al. Incidence of grade 3–4 adverse events, dose reduction, and treatment discontinuation in castration-resistant prostate cancer patients receiving PARP inhibitors: a meta-analysis. Expert Opin Drug Metab Toxicol. 2022;18(3):235–240. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CJ, Smith MR, de Bono JS, et al. ; for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain MHA, Kocherginsky M, Agarwal N, et al. BRCAAWAY: A randomized phase 2 trial of abiraterone, olaparib, or abiraterone+ olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) with DNA repair defects. J Clin Oncol. 2022;40(16 suppl):5018. [Google Scholar]
- 39.Hussain M, Daignault S, Twardowski P, et al. Abiraterone + prednisone (Abi) +/− veliparib (Vel) for patients (pts) with metastatic castration-resistant prostate cancer (CRPC): NCI 9012 updated clinical and genomics data. J Clin Oncol. 2017;35(15 suppl):5001. [Google Scholar]
- 40.Anampa-Guzman AC, Sulca-Huamani O, Perez-Mendez R, et al. Prognostic factors for overall survival in patients with metastatic castration-resistant prostate cancer: secondary analysis. J Clin Oncol. 2017;35(6 suppl):e597. [Google Scholar]
- 41.Markowski MC, Antonarakis ES. BRCA1 Versus BRCA2 and PARP Inhibitor Sensitivity in Prostate Cancer: More Different Than Alike? J Clin Oncol. 2020;38(32):3735–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohyuddin GR, Aziz M, Britt A, et al. Similar response rates and survival with PARP inhibitors for patients with solid tumors harboring somatic versus Germline BRCA mutations: a Meta-analysis and systematic review. BMC Cancer. 2020;20(1):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad F, Armstrong AJ, Thiery-Vuillemin A, et al. PROpel: phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2022;40(6 suppl):11. [Google Scholar]
- 44.Agarwal N, Azad A, Carles J, et al. TALAPRO-2: phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(6 suppl):LBA17. [Google Scholar]
- 45.Olmos D, Lorente D, Alameda D, et al. Presence of somatic/germline homologous recombination repair (HRR) mutations and outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) receiving first-line (1L) treatment stratified by BRCA status. Journal of Clinical Oncology. 2023;41(16):5003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


