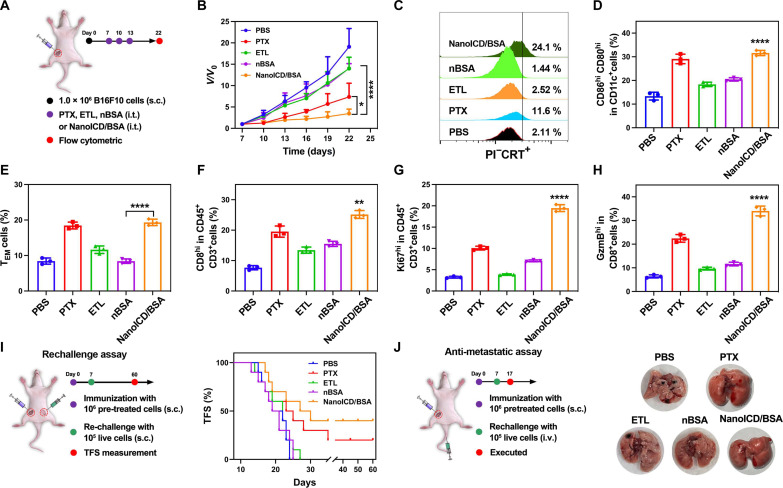
Fig. 4. NanoICD activates ICD-associated antitumor immunity in vivo.
(A) Schematic illustration of the experimental design. (B) Average tumor growth kinetics in different groups (PBS, PTX, ETL, nBSA, and NanoICD/BSA). (C) Pre-apoptotic CRT exposure on tumors from mice treated with PBS, ETL, PTX, nBSA, and NanoICD/BSA. (D and E) The population of CD80+CD86+ DC cells (gated on CD11c+) in tumor-draining lymph nodes (D) and CD44+CD62L− effector memory cells (gated on CD3+CD8+) in spleen (E) from mice treated with PBS, PTX, ETL, nBSA, and NanoICD/BSA. (F to H) The population of TILs (F), Ki67+CD3+ (gating on CD45+) (G), and GzmB+CD8+ (gating on CD45+CD3+) (H) in tumors from mice treated with PBS, PTX, ETL, nBSA, and NanoICD/BSA. (I) Schematic illustration of the establishment of the vaccination model to evaluate the ability of NanoICD/BSA to against tumor recurrence (left), and the tumor free survival (TFS) of C57BL/6 mice after inoculation of pre-treated B16F10 cells (PBS, PTX, ETL, nBSA, or NanoICD/BSA for 24 hours), followed by the inoculation of live B16F10 cells at the contralateral flank, n = 10 (right). (J) Schematic illustration of the establishment of the vaccination model to evaluate the ability of NanoICD/BSA to against tumor lung metastases (left), and the lung metastases in mice after the indicated treatments (right). Data are presented as means ± SD from six independent experiments for (B) (biological replicates, n = 6) and three independent experiments for (D) to (H) (biological replicates, n = 3). Significant levels are *P < 0.05, **P < 0.01, and ****P < 0.0001.