Abstract
Historically, clinical evaluation of unresponsive patients following brain injury has relied principally on serial behavioral examination to search for emerging signs of consciousness and track recovery. Advances in neuroimaging and electrophysiologic techniques now enable clinicians to peer into residual brain functions even in the absence of overt behavioral signs. These advances have expanded clinicians’ ability to sub-stratify behaviorally unresponsive and seemingly unaware patients following brain injury by querying and classifying covert brain activity made evident through active or passive neuroimaging or electrophysiologic techniques, including functional MRI, electroencephalography (EEG), transcranial magnetic stimulation-EEG, and positron emission tomography. Clinical research has thus reciprocally influenced clinical practice, giving rise to new diagnostic categories including cognitive-motor dissociation (i.e. ‘covert consciousness’) and covert cortical processing (CCP). While covert consciousness has received extensive attention and study, CCP is relatively less understood. We describe that CCP is an emerging and clinically relevant state of consciousness marked by the presence of intact association cortex responses to environmental stimuli in the absence of behavioral evidence of stimulus processing. CCP is not a monotonic state but rather encapsulates a spectrum of possible association cortex responses from rudimentary to complex and to a range of possible stimuli. In constructing a roadmap for this evolving field, we emphasize that efforts to inform clinicians, philosophers, and researchers of this condition are crucial. Along with strategies to sensitize diagnostic criteria and disorders of consciousness nosology to these vital discoveries, democratizing access to the resources necessary for clinical identification of CCP is an emerging clinical and ethical imperative.
Keywords: coma, cognitive-motor dissociation, vegetative state, minimally conscious state, philosophy of mind, ontology
“...As unconscious processes approach nearer the threshold of consciousness they take on more and more the qualities and attributes of conscious processes... Naturally the boundary lines between these subdivisions must constantly be shifting within a relatively narrow range... characteristic of disease is a wide excursion and irregularity of these boundary lines...”
Introduction: From Behavior to Brain Activity for Probing Consciousness in the Injured Brain
Covert cortical processing (CCP) is an emerging and clinically relevant disorder of consciousness (DoC) marked by the presence of intact association cortex responses to environmental stimuli in the absence of behavioral evidence of stimulus processing. Historically, clinical evaluation of unresponsive patients following brain injury has relied principally on serial behavioral examination to search for emerging signs of consciousness (i.e. capacity for subjective experience) and track recovery (Aparicio and Christos 2023, Young 2023). Patients who did not exhibit signs of wakefulness or awareness were dubbed comatose, and patients with wakefulness but without awareness dubbed vegetative (PVS Multisociety Task Force 1994). This canonical clinical approach has a long philosophical and neuroscientific history, reflected in Wittgenstein’s remarks in Philosophical Investigations (1953) that “there can be no identification of the mental without acknowledging the constitutive behavioral criteria for psychological attributes … ascribability of a psychological attribute to a creature is constrained by the creature’s behavioral repertoire” (Hacker 2018), and later codified by Plum and Posner in their magnum opus on the Diagnosis of Stupor and Coma (Plum and Posner 1982).
Advances in neuroimaging and electrophysiologic techniques now enable clinicians to lift the veil of behaviors, peering beyond into residual brain functions even in the absence of overt behavioral signs. Here, we allude to Schopenhauer’s interpretation of the ‘veil of Maya’ to illustrate the idea that traditional behavioral assessments and corresponding nomenclature may not fully capture underlying states of consciousness in patients with DoC. In The World as Will and Representation, Schopenhauer’s remarks that the veil of Maya “covers the eyes of mortals, and causes them to see a world of which one cannot say either that it is or that it is not… like the sunshine on the sand which the traveler from a distance takes to be water, or like the piece of rope on the ground which he regards as a snake… But what all these meant, and that of which they speak, is nothing else but what we are now considering, namely the world as representation subordinated to the principle of sufficient reason” (Schopenhauer 1969).
In lifting this proverbial veil, technological advances have expanded clinicians’ ability to sub-stratify behaviorally unresponsive and seemingly unaware patients following brain injury by querying and classifying covert brain activity made evident through active or passive neuroimaging or electrophysiologic techniques, including functional MRI (fMRI), electroencephalography (EEG), transcranial magnetic stimulation-EEG (TMS-EEG), and positron emission tomography (PET). These modalities may be used to not only measure a patient’s level or state of consciousness (e.g. comatose, vegetative state (VS)/unresponsive wakefulness syndrome (UWS), or minimally conscious state (MCS)) and capacity for recovery, but increasingly are beginning to be used to query the content of a patient’s consciousness, including imagined speech and scenes, in the absence of overt interaction (Scotti et al. , Sorger and Goebel 2020, Daly 2023, Giraud and Su 2023, Ozcelik and VanRullen 2023, Tang et al. 2023, Willett et al. 2023, Wilson et al. 2023). Clinical research has thus reciprocally influenced clinical practice, giving rise to new diagnostic categories including cognitive- motor dissociation, (CMD, i.e. ‘covert consciousness’) (Schiff 2015) and CCP (Edlow et al. 2021). While covert consciousness (i.e. command-following) has received extensive attention and empiric study, CCP is relatively less understood. Patients previously grouped together within more coarse, behavior-based diagnostic categories may now be reclassified or subclassified with greater precision based on the nature and extent of these advanced findings (Kondziella et al. 2021).
The expansion of consciousness-related nosology made possible through novel neurotechnologies is increasingly recognized by clinicians, ethicists, and philosophers (Del Pin et al. 2021, Young et al. 2021, Seth and Bayne 2022). Yet, there is great uncertainty about how to define and operationalize these emerging diagnostic categories in clinical practice, how pre-existing diagnostic approaches predicated on the presence or absence of overt behaviors should be accordingly revised or jettisoned, and what the phenomenological correlates of these newly recognized states might be. In this article, we describe and critically evaluate these timely and underexplored issues and offer formative guidance on how to harmonize approaches to defining and integrating states of covert brain activity in clinical practice and research.
The emergence of covert brain activity: looking back and looking forward
In a pioneering 1970 study entitled “The blood flow and oxygen consumption of the dying brain”, neurosurgeon Dr. Mordechai Shalit and colleagues described one of the first efforts to identify quantifiable measures of brain activity correlating with states of consciousness and its recovery or deterioration following acute severe brain injury (Fig. 1). Their study, drawing on discoveries made over the preceding several decades linking cerebral blood flow (CBF), metabolism, and function (Roy and Sherrington 1890, Schmidt 1928a, 1928b, Kety and Schmidt 1948, Kety 1950), examined brain metabolic activity as reflected by CBF and oxygen consumption measured directly via angiography and blood sampling in nine patients with acute coma following brain injury. The investigators correlated these measures with “the appearance of the accepted clinical phenomena indicating brain functional level, such as the response to external stimuli, muscle tone, respiration, blood pressure, and EEG, in patients with severe brain damage” (Shalit et al. 1970). In describing the motivation for this study, Shalit and colleagues presciently remarked that “evaluation of the clinical status and prognosis of comatose patients is, in many cases, extremely difficult. In patients existing between…extremes, where brain activity is still preserved, although at subnormal levels, it may be impossible to distinguish between the patient who will die within a short time and the one who will recover completely. Both may seem clinically similar… yet one of them has a brain which has been severely and irreversibly injured while the other suffers only a transient functional loss [and] have led to a need for an objective, precise, and rapid evaluation of the brain’s condition in the race with time. Cerebral oxygen consumption, being the most important factor indicating brain metabolic activity in normal conditions, was thought to be an appropriate representation of the brain’s functional status...Some vitality may still be hidden and preserved in deep brain structures… cerebral pathological processes that conceal themselves behind the apparently stable clinical status … are still totally unknown to us, and new methods should be used for their investigation.” The prognostic utility of total and regional CBF measurements was later corroborated by findings of William Heiss et al. in 1972 (Heiss et al. 1972).
Figure 1.
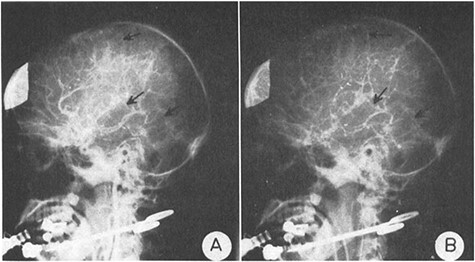
Historical figure from Shalit et al. 1970. Shalit et al. examined brain metabolic activity as reflected by CBF and oxygen consumption measured directly via angiography and blood sampling in nine patients with acute coma following brain injury
In a 1974 follow-up study entitled “Clinical equivalents of cerebral oxygen consumption in coma”, Shalit and colleagues described their team’s subsequent efforts to identify among 24 patients with coma following severe brain injuries of varied etiologies “the lowest value [of cerebral oxygen consumption] above which coma might still be reversible and the highest value below which coma is always irreversible and the patient either remains in a chronic VS or eventually dies” (Shalit et al. 1972). Critical values of cerebral metabolic activity as reflected by oxygen consumption were identified that could help differentiate reversible from irreversible coma. These efforts set the stage for subsequent decades of investigation wielding cutting-edge techniques to probe patients’ capacity for recovery of consciousness (understood as capacity for subjective experience) beyond overt bedside signs.
In a groundbreaking symposium organized in 1974 in London and published in 1975 by Fred Plum on “Outcome of Severe Damage to the Central Nervous System,” David Ingvar and Manuel Ciria reviewed these earlier findings and presented results of their innovative effort to measure regional CBF (rCBF) in 35 patients with severe brain injury using a novel intra-arterial xenon-133 method. These measures were performed during rest and “sensory activation” conditions, including sensory stimulation, acoustic activation (calling patient’s name), and photic activation (intermittent light stimulation). Leveraging techniques refined over the preceding decade, Ingvar and Ciria generated topographic displays (Risberg and Ingvar 1972, 1973, Ingvar and Schwartz 1974) of regions activated under various conditions and painstakingly compared their findings with clinical features (Fig. 2) (Ingvar and Ciria 1975). Results revealed substantial variation in CBF patterns even among patients with similar behavioral semiologies, thus providing a more granular view of brain function beyond behavioral capabilities, and suggesting that “measuring rCBF enables us to assess severe damage to the central nervous system quantitatively and also to estimate whether higher functions are retained in severely reduced patients in coma, stupor, and apallic state—patients who more or less completely lack behavioral responses…. [and] offer a new possibility to test whether these patients have cerebral reactions of the type found normally during mental activity.” Foreshadowing vigorous ethical dialogue that would emerge decades later (Fins 2015, Young et al. 2021), Ingvar and Ciria emphasized the ethical importance of their findings, “[t]he fact that cerebral reactions indicating conscious perception can be recorded in the absence of behavioral signs of consciousness, is of immediate clinical and indeed humanitarian interest, and it suggests a much further use of rCBF studies in tragic cases of this type, with loss of behavior, not loss of consciousness, in which there is a suspicion of remaining conscious perception….This question is often difficult to answer merely on the basis of clinical observations especially in patients more or less completely lacking behavioral reactions” (Ingvar and Ciria 1975).
Figure 2.
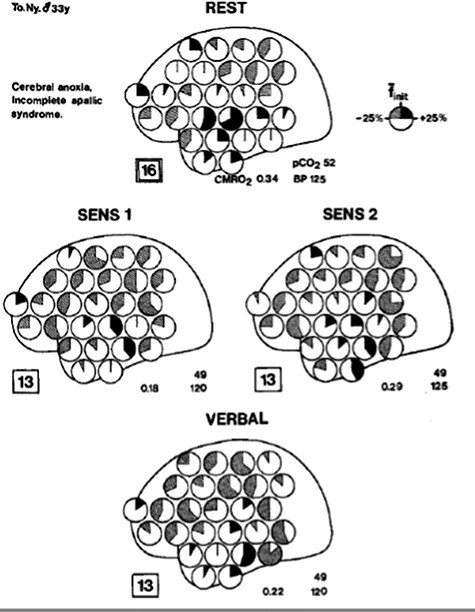
Historical figure from Ingvar et al. 1975 illustrating regional cerebral blood flow following sensory stimulation in patients following anoxic brain injury
In 1987, David Levy and colleagues at Cornell School of Medicine in New York and Montreal Neurological Institute carried out one of the first studies utilizing PET in brain-injured patients with the aim of discerning objective signatures of consciousness (Levy et al. 1987). In their study, entitled “Differences in CBF and Glucose Utilization in Vegetative Versus Locked-in Patients”, the authors described their motivation as stemming from an evolving recognition that the “distribution and severity of postmortem anatomical damage to the cerebral hemispheres can vary considerably [among patients in a VS], making it difficult to infer on pathological grounds alone whether or not self-awareness was preserved during life. Such uncertainty can be resolved only by additional objective measures of brain function in these patients” (Levy et al. 1987). By comparing PET measurements of rCBF and glucose metabolic rate of 7 patients in VS to those from 3 patients with locked-in syndrome (LIS), the investigators identified significant reductions in metabolic activity in the cortex, cerebellum, thalamus, and basal ganglia in patients with VS (Fig. 3), providing some of the first evidence that residual brain activity revealed through neuroimaging could serve as a “mark of the mental.”(Rorty 1970, Levy et al. 1987)
Figure 3.
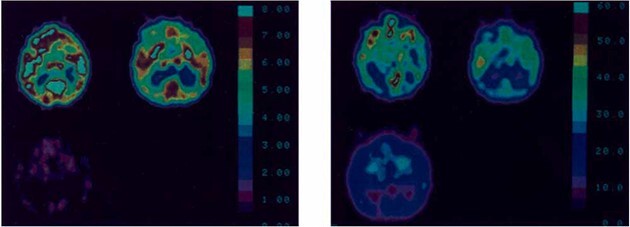
Historical figure from Levy et al. 1987 describing patterns of cerebral metabolism in patients following brain injury using brain PET imaging
These early studies presaged work by B.M. de Jong and team at University Hospital Groningen in the Netherlands nearly a decade later which for the first time applied a stimulus-based neuroimaging paradigm to query “preserved cortical circuitry involved in (rudimentary) cognitive function” (de Jong et al. 1997). These investigations of patients who appeared to be in a VS following brain injury were motivated by “the question of the parents whether the patient would notice their voice” (de Jong et al. 1997). “[T]he question suggests itself,” de Jong and colleagues asked, “whether some level of awareness might still be present of which expression is obstructed by complete paralysis” (de Jong et al. 1997). In their landmark 1997 case report entitled “Regional CBF changes related to affective speech presentation in persistent VS [PVS],” de Jong and colleagues played a tape-recorded familiar story told by the patient’s mother while measuring changes in rCBF by PET as compared to those measured during the presentation of non-verbal sound in a 16 year-old patient in apparent VS following severe brain injury (Fig. 4) (de Jong et al. 1997). Remarkably, significant activation was detected in the right temporal, anterior cingulate, and premotor cortices in the story stimulus condition, reflecting appropriate cortical responses in language processing brain regions. The authors boldly concluded that “in some circumstances of [apparent] PVS, elements of complex stimulation may be processed in appropriate cortical circuitry…[and] suggest that the distinction between PVS and LIS may be a gradual one” (de Jong et al. 1997). These findings provided the first clear empirical support for the hypothesis that higher order cortical processing in response to a salient stimulus might be covertly preserved in a behaviorally unresponsive patient presumed to be unconscious on the basis of the bedside neurological exam—the first evidence of what was later called CCP (Edlow et al. 2021).
Figure 4.
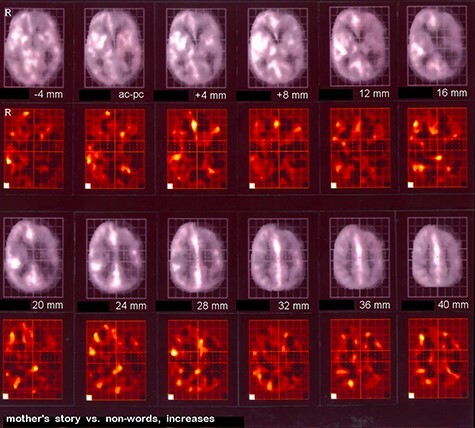
Historical figure from B.M. de Jong et al. 1997 demonstrating regional cerebral blood flow changes related to affective speech stimulation to a patient considered to be in the vegetative state
The following year, David Menon and colleagues at the Wolfson Brain Imaging Centre Team at the University of Cambridge published a case report entitled “Cortical processing in persistent VS” which used PET imaging to study what was then dubbed “covert cognitive processing” in response to presentation of familiar faces displayed to a 26 year-old patient in an apparent VS four months following onset of acute disseminated encephalomyelitis (Menon et al. 1998). Menon et al. identified significant activation in the right fusiform gyrus, dorsal cerebellum, and extrastriate areas (Fig. 5), concluding that “she not only perceived visual stimuli, but also processed them to recognize content that was not based on primary image attributes such as color, brightness, size, or movement” (Menon et al. 1998). Two months following this study, the patient regained behavioral responsiveness, overt facial recognition, and language capacity (Menon et al. 1998).
Figure 5.
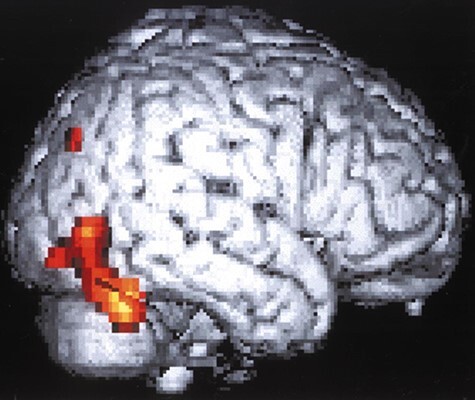
Historical figure from Menon et al. 1998 which used PET imaging to study brain responses to familiar faces in a behaviorally unresponsive patient
Later that year, Urs Ribari and colleagues at Cornell School of Medicine applied magnetoencephalography for the first time to record spontaneous and evoked magnetic potentials and extracted gamma-band oscillations following auditory and somatosensory stimulation in five patients considered to be in PVS, concluding that “global integration of modular functions characterizes the normal human brain but [some seemingly] unconscious persons [in PVS] may express partially preserved modules of activity” (Ribary et al. 1998).
In 2000, building upon these precedents, Steven Laureys and colleagues at University of Liege completed a PET study of five patients in apparent VS to assess cerebral responses to auditory stimulation (Laureys et al. 2000). They identified activation of primary auditory cortices in patients with VS (Fig. 6), but notably (and differing from the earlier findings reported by de Jong and colleagues) did not find significant activation in hierarchical multimodal associative areas including the temporoparietal junction of the superior temporal sulcus, with observed functional disconnection from the posterior parietal associative area, anterior cingulate, and hippocampus (Laureys et al. 2000). However, the auditory stimulus used in this study was rudimentary, consisting of monoaural clicks and contralateral white noise, in contrast to the more salient, language-based auditory stimulus (a familiar story told by patient’s mother) utilized by de Jong several years prior. The retrospective juxtaposition of these seminal studies and their varied findings relative to different stimulation paradigms foreshadow a later development in the field toward the use of hierarchical paradigms to assess differential responses to progressively richer varieties of stimuli (Coleman et al. 2009a).
Figure 6.
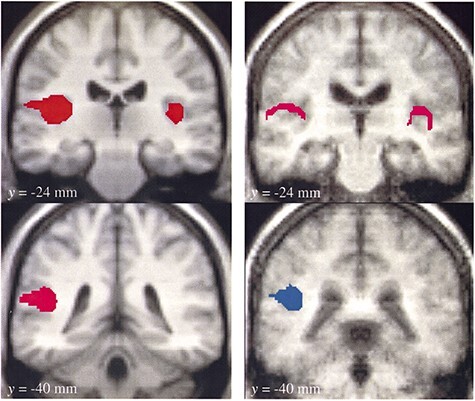
Historical figure from Laureys et al. 2000 examining PET responses in brains of patients in behavioral vegetative state to auditory stimulation
From auditory processing to somatosensory processing
In 2002, Laureys’ team performed PET in 15 patients in apparent VS to measure cortical responses to noxious somatosensory stimulation (high-intensity median nerve electrical stimulation) (Laureys et al. 2002). Despite absent EEG cortical evoked potentials, they identified significant PET activation in the midbrain, thalamus, and primary somatosensory cortex in every patient; however, no activation was seen in the secondary somatosensory, insular, posterior parietal, and anterior cingulate cortices, with additional functional disconnection noted with premotor, polysensory superior temporal, and prefrontal cortices (Fig. 7), indicating that, in these patients “activation of primary sensory cortex seems to subsist as an island, dissociated from higher-order cortices” with the essential caveat that “[i]n the absence of a generally accepted neural correlate of pain and consciousness, it is difficult to make definite judgments about awareness in PVS patients” (Laureys et al. 2002).
Figure 7.
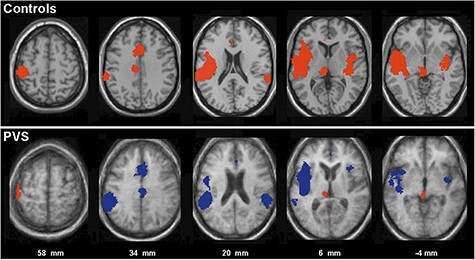
Historical figure from Laureys et al. 2002 examining PET responses in brains of patients in behavioral vegetative state to noxious somatosensory stimulation
From auditory to semantic processing and from neuroimaging to electrophysiology
In 2006, Fabien Perrin and colleagues contrasted EEG-based auditory evoked potentials to the patient’s own first name with responses to others’ first names (Perrin et al. 2006). Using this method, Perrin et al. explored differential abilities of patients with disorders of consciousness to discriminate meaningful/salient words (patient’s own name), indicated by the emergence of a P300 wave (Fig. 8) (Perrin et al. 2006). The P300, or P3, is an evoked EEG potential occurring approximately 300 milliseconds following the presentation of a particularly relevant, meaningful, or task-relevant stimulus, and most strongly detected by electrodes covering the parietal lobe (hence P300). The P300 potential is thought to reflect allocation of attentional or information-processing cognitive resources and in 1995 was found by Hillel Pratt and Idan Berlad to reliably reflect healthy subjects’ processing of their own name (Chapman and Bragdon 1964, Wickens et al. 1983, Berlad and Pratt 1995). Of the 15 patients studied by Perrin et al., 5 were in apparent VS, 6 in MCS, and 4 were affected by LIS (Perrin et al. 2006). As expected, all of those in LIS and MCS demonstrated intact P3, however remarkably, 3 apparent VS patients demonstrated a preserved P3, indicating that at least some patients diagnosed as being in VS may harbor “partially preserved, albeit restricted, cerebral processing for “automatic speech comprehension” and that “islands of cerebral function may be preserved in some—but not all—VS patients” (Perrin et al. 2006) Notably, P300 waveforms can be variable and are subject to numerous factors, including the individual’s state of arousal, attention, and possibly other uncontrolled factors. Thus, the presence of a P300 waveform, while indicative of certain processing activities, does not definitively confirm conscious awareness Daltrozzo et al. (2007).
Figure 8.
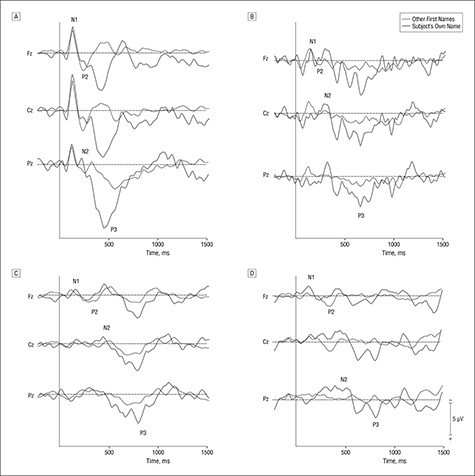
Historical figure from Perrin et al. 2006 examining EEG-based auditory to identify preserved speech comprehension among patients with disorders of consciousness
These findings were among the first to challenge the prevailing clinical wisdom that all patients diagnosed as VS categorically lack sentience of their environment, as there was now evidence that at least some VS patients exhibit reliable cortical responses in a contingent manner to salient sensory stimuli. However, the phenomenological significance of these findings, particularly whether they reflect consciously experienced sensory perception or if they occur automatically and without a phenomenological correlate, could not be definitively answered; indeed, P300 waveforms have been identified in conditions of subliminal stimuli in addition to supraliminal stimuli, suggesting that meaningful stimuli may be perceived and processed seemingly subconsciously, without reportable awareness of the perceptual information presented, even among healthy individuals (Brázdil et al. 2001, Di et al. 2007, Wang et al. 2015). Figuring prominently in the interpretation of such findings are challenges in inferring comprehension and subjective experience from passive paradigms, where patients are not asked to actively respond to a cognitive probe. As we will later explore, difficulties in discerning what a patient is awaringly understanding versus reflexively or unconsciously processing under passive paradigms may be addressed through the use of increasingly complex passive stimuli. However, further phenomenological and translational neuroscience research is essential to elucidate which signatures of brain processing elicited through passive stimuli are conclusively dispositive of conscious awareness (Kronemer et al. 2022).
Imputing intentionality in the era of fMRI
That same year, Adrian Owen et al. leveraged advances in task-based fMRI to demonstrate language comprehension and volitional modulation of brain activity in a patient diagnosed as being in a chronic VS (Owen et al. 2006). The investigators asked the patient, while in the MRI scanner, to imagine playing tennis and imagine navigating her house and strikingly observed localized blood-oxygen-level-dependent changes indicating covert language comprehension and command following (Fig. 9) (Owen et al. 2006). Owen et al. boldly concluded that “despite fulfilling the clinical criteria for a diagnosis of VS, this patient retained the ability to understand spoken commands and to respond to them through her brain activity, rather than through speech or movement… her decision to cooperate with the authors by imagining particular tasks when asked to do so represents a clear act of intention, which confirmed beyond any doubt that she was consciously aware of herself and her surroundings” (Owen et al. 2006). This finding augured a wave of redoubled efforts to develop and deploy methods that bypass the potentially injured efferent motor system in severely brain-injured patients to probe preserved cognitive function more directly and in a way that would not hinge on a patient’s overt behavioral repertoire, which, as data were beginning to suggest, could at times be dissociated from a patient’s underlying cognitive function. An emerging diagnostic entity, cognitive-motor dissociation (i.e. covert consciousness), thus began to surface in clinical consciousness, but over a decade would elapse until medical professional societies began to recognize a role for advanced neuroimaging and electrophysiologic techniques in the clinical evaluation of behaviorally unresponsive patients following brain injury.
Figure 9.
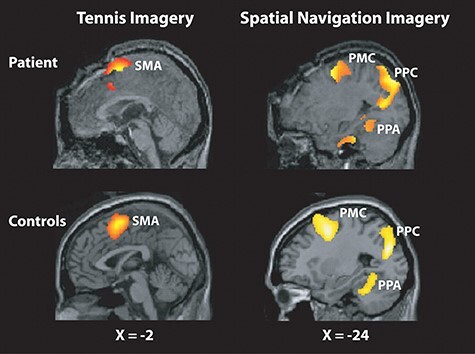
Historical figure from Owen et al. 2006 identifying volitional modulation of brain activity in patient diagnosed as being in chronic vegetative state
The following year, in 2007, Martin Coleman et al. applied a hierarchical auditory processing fMRI paradigm to probe and disambiguate multiple potential levels of passive language processing in brain-injured patients—(1) auditory (as measured by responses to noise versus silence), (2) perceptual (as measured by responses to speech versus unintelligible noise), and (3) semantic (as measured by responses to semantically ambiguous speech versus semantically unambiguous speech) (Coleman et al. 2007). Of 7 patients in the study who met diagnostic criteria for VS, 3 demonstrated intact auditory and perceptual responses and 2 remarkably demonstrated intact auditory, perceptual, and semantic responses that were anatomically appropriate and comparable to those seen in healthy control individuals (Fig. 10) (Coleman et al. 2007). Consistent with findings from prior studies, the authors concluded that “a small number of patients with a diagnosis of VS may retain islands of residual cognitive function that cannot be observed using methods that rely on the patients’ ability to make overt motor responses… and that in the absence of behavioral evidence, functional imaging provides a valuable tool to the assessment eam.” Coleman and colleagues corroborated these findings in a subsequent and larger 2009 study provocatively entitled “Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness,” (Coleman et al. 2009b) which additionally demonstrated for the first time the prognostic significance of covert speech processing, indicated by improved neurobehavioral outcomes on the Coma Recovery Scale—Revised 6 months later (Fig. 11) (Giacino et al. 2004, Coleman et al. 2007). While it is remarkable that patients diagnosed with VS showed responses akin to healthy control participants, it is important to note that these responses are not definitive indicators of conscious awareness or comprehension, as responses in the auditory, perceptual, and semantic domains may occur without conscious perception.
Figure 10.
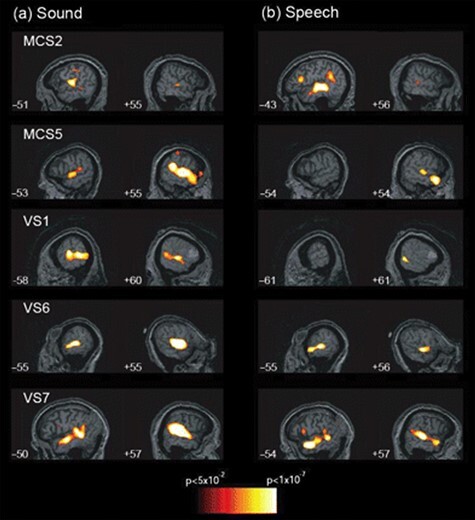
Historical figure from Coleman et al. 2007 identifying hierarchical auditory processing in some patients with a diagnosis of vegetative state following brain injury
Figure 11.
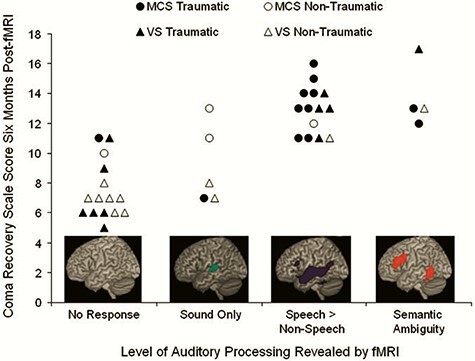
Historical figure from Coleman et al. 2009b demonstrating prognostic significance of covert speech processing in patients with disorders of consciousness
In light of emerging evidence of diagnostic and prognostic significance, coupled with growing knowledge of independent shortcomings of the standard behavioral examination (Andrews et al. 1996, Schnakers et al. 2009), a flurry of studies followed using resting-state, passive stimulus-based, and active task-based neuroimaging and electrophysiologic paradigms, independently or together, to improve the diagnosis of consciousness and prediction of its recovery among patients with chronic brain injury lacking self-expression (Monti et al. 2010, Cruse et al. 2011, Goldfine et al. 2011, Okumura et al. 2014, Stender et al. 2014, Fernández-Espejo et al. 2015, Braiman et al. 2018, Gui et al. 2020, Walter and Hinterberger 2022). Increasing awareness of the high false-negative rates of cognitively burdensome active paradigms further underscored the importance of including passive paradigms in multimodal diagnostic approaches (Cruse et al. 2011, Edlow et al. 2017).
The puzzles and paradoxes brought into sharp focus by the possibility of discordance between patients’ levels of behavioral responsiveness and conscious awareness began to be explored by neurologist and ethicist James L Bernat, who writing in Neurology in 2002 emphasized that it is “[a]t the bedside, one can only crudely measure evidence of the fullness of human awareness. So how can we be certain that the awareness of patients in MCS is minimal? Given that the criteria for MCS measure impaired responsiveness, perhaps it would be more accurate to use the older term “minimally responsive” to describe them. An error that clinicians commonly make is underestimating the degree of a severely disabled patient’s awareness when that patient’s responses to stimuli are deficient” (Bernat 2002). Indeed, as Bernat alluded to, earlier “Recommendations for Use of Uniform Nomenclature Pertinent to Patients With Severe Alterations in Consciousness” of the American College of Rehabilitation Medicine in 1995 used the term minimally responsive state rather than MCS, explicitly recognizing that “considerable confusion and controversy on the use of diagnostic and clinical terms assigned to patients with severe alterations in consciousness… results largely from the lack of a uniform classification system that is based on behaviorally defined criteria,” (Giacino et al. 1995). Echoing and amplifying many of these challenges, in 2017 Lionel Naccache proposed that the term “cortically mediated state” be used in lieu of the term MCS in response to accumulating evidence that the umbrella of MCS problematically includes a “large and heterogeneous set of states that may span from unconscious patients with residual islets of cortical activity that translates into overt behavior, to conscious but cognitively impaired patients that may be self-conscious but unable to go from preserved response to command to the functional use of a communication code, due to executive deficits (working memory, executive control)” (Naccache 2018). Underscoring the conceptual challenges in interpreting the diagnosis of MCS, Naccache proffered that “the adverb ‘minimally’ in ‘MCS’ is rather dubious and introduces some perplexity for theorists, clinicians, caregivers, and patients’ relatives. What is really ‘minimal’ in MCS: consciousness, state definition or stability over time … or simply our understanding?” (L. Naccache 2018). Despite these conceptual shortcomings, expounded upon by philosophers and clinicians alike, (Bayne et al. 2018, L Naccache 2018, Lazaridis 2019, Hermann et al. 2021), prevailing clinical nomenclature has remained largely unchanged, (Choi and Young 2023, Golden et al. 2023) with some rebutting the nomenclature shift from MCS to cortically mediated state proposed by Naccache on philosophical and ethical grounds (Bayne et al. 2018).
Deciphering consciousness and cortical processing in the intensive care unit
For the first time applying some of these techniques in the acute setting, in 2017, our group at Massachusetts General Hospital published a study that prospectively applied both task-based and stimulus-based fMRI and EEG to evaluate behaviorally unresponsive patients in the ICU following acute traumatic brain injury (Fig. 12) (Edlow et al. 2017). Profoundly consequential decisions made in the intensive care unit (ICU) often revolve around patients’ level of consciousness and likelihood of recovery, yet prior to this most efforts to detect consciousness using advanced assessments had focused on patients in chronic stages, limiting generalizability to this highly urgent and actionable context where pivotal decisions to continue, limit, or withdraw life-sustaining treatments are initially made. Efforts to improve identification of consciousness in the ICU carry immense and often life-or-death consequences for patients. This study incorporated both active task-based paradigms (motor imagery task) as well as passive stimulus-based paradigms (presentation of language and music stimuli). To classify results, CMD was characterized by demonstration of covert command-following evidenced by modulation of brain activity during active task paradigms, whereas higher-order cortex motor dissociation (conceptually a homologue of CCP) was introduced as a new refinement in nomenclature characterized by demonstration of association cortex responses during passive language or music paradigms (e.g. response in Wernicke’s area during language paradigm and not just in Heschel’s gyrus), despite the absence of behavioral signs of environmental awareness. Of 16 patients, CMD was detected in 4 patients, and higher-order cortex motor dissociation was identified in 2 additional patients. The prevalence and prognostic relevance of CMD in acute brain injury were further clarified in studies by Jan Claassen and colleagues in 2019 and 2022, who identified CMD among 15% of behaviorally unresponsive patients with acute brain injury, with improved functional outcomes at 12 months as compared to those without CMD (Claassen et al. 2019, Egbebike et al. 2022).
Figure 12.
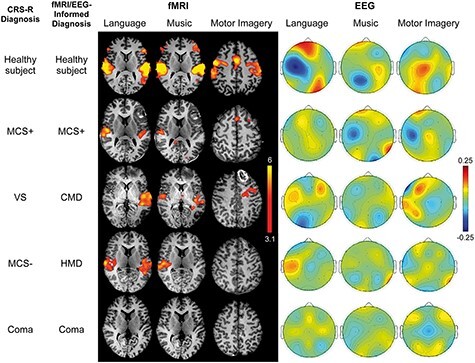
Historical figure from Edlow et al. 2017 illustrating task-based and stimulus-based fMRI and EEG response patterns in patients with acute disorders of consciousness in the intensive care unit following brain injury
In 2021, Sokoliuk et al. implemented a refined hierarchical language paradigm using EEG (Gui et al. 2020) to identify cortical signatures of dynamic language processing indicative of level of residual consciousness in behaviorally unresponsive patients following brain injury, also finding that preserved processing predicts recovery (Fig. 13) (Sokoliuk et al. 2021). “Covert speech comprehension” accordingly emerged as yet another diagnostic term to classify patients who demonstrate neurophysiologic tracking of high-level language structures but who lack bedside behavioral evidence of language comprehension, building upon prior methodological insights (Owen et al. 2005, Coleman et al. 2007, Beukema et al. 2016, Sokoliuk et al. 2021). However, difficulties remained in conclusively inferring comprehension from brain responses to passive stimuli (Edlow and Naccache 2021). Later that year, Edlow and colleagues proposed the more general diagnostic term CCP to refer to association cortex responses to passive sensory stimuli in patients who do not show evidence of such perceptual function on the bedside behavioral examination (Edlow et al. 2021).
Figure 13.
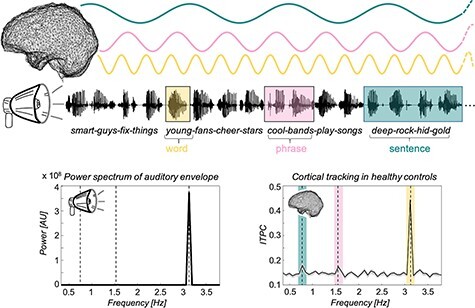
Historical figure from Sokoliuk et al. 2021 illustrating hierarchical language paradigm using EEG to identify signatures of language processing indicative of residual consciousness following acute brain injury
Covert command-following demonstrated via responses to prompts that can be detected on fMRI and EEG provides near definitive evidence of preservation or recovery of key cognitive processes including language comprehension, basic attention, memory, and expression. However, a high false negative rate, wherein fMRI and EEG fail to detect covert command-following in up to 25% of control subjects with behavioral command-following intact, (Cruse et al. 2011, Edlow et al. 2017) makes this approach unappealing as a highly sensitive and specific diagnostic test. Covert command-following tasks are cognitively burdensome, could result in variable patterns of fMRI and EEG signals, and rely on intact domains of cognition that likely exceed what is necessary for consciousness (Claassen et al. 2023). On the other hand, passive paradigms do not rely on demanding cognitive tasks and may be more robust to intra-subject variability and confounding due to cognitive impairments and fluctuations in arousal.
Perturbational complexity index and the capacity for consciousness
As described in previous sections, the ability to consistently express behavioral evidence of language or motor function or to volitionally modulate the brain activity when asked to perform a task is considered evidence for the presence of consciousness. However, their absence is not typically considered evidence of unconsciousness. For example, dreaming and hallucinations are forms of disconnected consciousness during which an individual has conscious experiences even in the absence of external inputs or motor behavior engagement. Other conditions including delirium, post-lesional confusional states, dissociative disorders, and psychosis may also reflect states of disconnected, disintegrated, or disordered consciousness that may benefit from theoretical harmonization and study, including testing the impact of these states on both attention-demanding and non-attention-demanding tests (Bhat and Rockwood 2007, Young 2018, Sherer et al. 2020, Berkovitch et al. 2021, Whiteley 2021a, 2021b, Kim et al. 2022, Tanabe et al. 2022). Indeed, the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-5) designates “disturbance in attention (i.e. reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment)” as necessary criteria of delirium (European Delirium Association and American Delirium Society 2014).
Accordingly, brain-based measures have been devised to quantify neural mechanisms that are relevant for consciousness independently of sensory processing, executive functions, and motor outputs. This approach, relying on the analysis of internal brain properties, is particularly relevant in patients with brain injury who have sustained cranial nerve, visual, sensory, motor, or auditory system trauma that may limit the reliability of task-based or stimulus-based paradigms. For example, language-based stimulus or task-based paradigms cannot yield informative results in a patient with acquired deafness as a result of bilateral acoustic nerve injury following blast trauma. In this context, measures designed to quantify neural complexity, defined as the joint presence of functional differentiation and functional integration in cortico-thalamic networks, are particularly promising as they are both grounded in general theoretical principles (Tononi and Edelman 1998, Mediano et al. 2022) and corroborated by converging empirical evidence (Sarasso et al. 2015). Among these, the perturbational complexity index (PCI), whereby brain complexity is assessed causally using direct cortical perturbations with TMS-EEG (Casali et al. 2013), shows promising diagnostic characteristics. PCI results in unprecedented specificity and sensitivity in discriminating conscious and unconscious conditions in benchmark conditions including disconnected states, such as dreaming and hallucinations (Sarasso et al. 2015, Casarotto et al. 2016, Bradley et al. 2022). Notably, PCI has been leveraged to stratify behaviorally unresponsive patients by identifying signatures of brain complexity compatible with capacity for consciousness following severe brain injury (Casarotto et al. 2016). Coalescing threads of theoretical and empirical research have begun to clarify the relevance of complexity to consciousness (Sarasso et al. 2021, Nemirovsky et al. 2023). As efforts to understand what CCP means continue apace, PCI promises to provide further granularity. However, more research is needed to explore the possible links between CCP and PCI. For instance, studies are needed to explore whether patients with CCP will generally have higher complexity responses (i.e. higher PCI values) as compared to patients without CCP. Some patients might demonstrate high complexity but due to severe sensory and motor impairments exhibit no other covert or overt signs of consciousness to suggest CCP or CMD; this state might be referred to as “covert brain complexity” (CBC). In those with known sensory or motor impairments, TMS-EEG PCI may thus prove to be a superior test as it bypasses sensory and motor pathways (which are often disrupted in patients with DoC) and causally probes thalamocortical network connectivity implicated in human consciousness (Lee et al. 2022, Edlow et al. 2023). Whether the brain’s capacity to sustain complex dynamics as indexed by PCI is a necessary condition for CCP has yet to be empirically studied, but is a theoretically plausible hypothesis.
Can consciousness (in the sense of having the capacity for subjective experience) be assumed when there is evidence of CCP or cortical complexity? There are a range of theoretical possibilities that could characterize this relationship—taking complexity and consciousness as an example, the association between consciousness and PCI can range from loose to strong:
(i) cortical complexity is neither necessary nor sufficient for consciousness [i.e. cortical complexity is a contingent epiphenomenon or defeasible proxy for consciousness].
(ii) cortical complexity is a sufficient but not necessary condition for consciousness [i.e. conscious states may exist in the absence of cortical complexity, but when complexity is present we can always infer that consciousness is present].
(iii) cortical complexity is a necessary but not sufficient condition for consciousness [i.e. some other ingredient(s) in addition to cortical complexity are required to generate conscious experience]—this presumed relationship between consciousness and PCI has the strongest evidentiary support (Tononi and Edelman 1998).
(iv) cortical complexity is a necessary and sufficient condition for consciousness [i.e. there cannot be consciousness without cortical complexity and there cannot be cortical complexity without consciousness].
(v) cortical complexity is identical to consciousness [e.g. as H2O is to water, cortical complexity is to consciousness].
Whether complexity is sufficient for consciousness is currently unknown, and drawing an identity relationship between brain based measures and consciousness is limited considering the spatiotemporal scale of empirical metrics. Yet, complexity measures appear one step closer to facilitating stratification of patients’ states of consciousness compared to other EEG-based indices. For example, the presence of alpha power on resting state EEG is typically considered an index of preserved brain function and consciousness. Nonetheless, alpha power can be found suppressed in conditions during which consciousness is present but disconnected (Carroll et al. 2023, Colombo et al. 2023) such as dreaming (Esposito et al. 2004), hallucinations (Esposito et al. 2004), and in LIS (Babiloni et al. 2010). On the other hand, the presence of delta activity is typically associated with unconscious states. Yet, the predominance of slow delta activity in spontaneous scalp EEG recordings has been reported during conscious states (Frohlich et al. 2021). In light of these findings, the higher sensitivity and specificity of PCI suggest that measures of brain complexity are a closer proxy of the neural correlates of global conscious states and may allow for more reliable stratification of disordered states of consciousness in patients. Accordingly, preliminary evidence suggests that high PCI values can indicate the presence of consciousness in unresponsive patients even in the presence of a severely abnormal EEG background (Comanducci et al. 2023).
Is the presence of brain complexity sufficient to predict behavioral and functional recovery in patients affected by disorders of consciousness? In this context, PCI may guide clinicians and researchers in designing personalized treatments or interventions aimed at restoring capacity for consciousness in patients with low PCI or to restore responsiveness to the external environment in patients with high PCI values (Edlow et al. 2023). Further philosophical, ethical, and empirical investigations are essential to advance knowledge in this area and to clarify the relationship between complexity and CCP, especially as these findings will play an important role in how to appropriately communicate with families and surrogates (Boegle et al. 2022, Shapiro-Rosenbaum and Jaffe 2023).
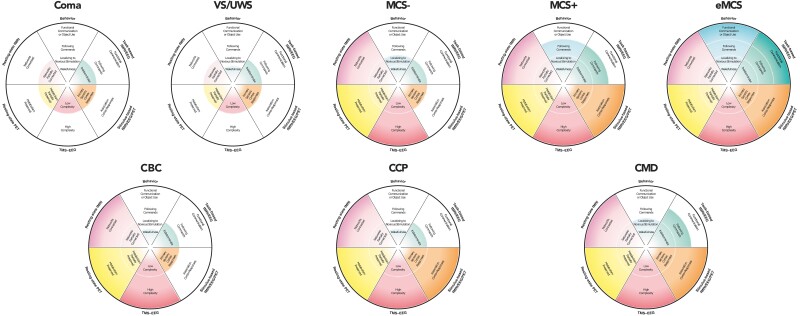
Current state of the art in CCP: towards a refined nosology
As methodologies continue to be refined and findings disseminated, a distinctive yet underexplored typology of consciousness and its clinical ascription has emerged. Within this emerging nosology, consciousness and its ascription exists along a spectrum and necessarily admits of degrees, supplanting diagnostic schemata that more coarsely classify consciousness as either present (eMCS), minimally present (MCS) or absent (VS/UWS/coma) along simple and often misleading behavioral lines.
Among behaviorally unresponsive patients, there are those whose cognitive status closely matches and is predicted by their behavioral status, but there are others whose cognitive status is decidedly dissociated from their behavioral status. Further, among behaviorally unresponsive patients with covert brain activity, there are some who demonstrate signs indicative of conscious awareness, as evidenced by covert command-following through volitional modulation of brain activity detected by task-based paradigms, yet others who, despite failing to demonstrate covert command-following, manifest preserved processing of salient stimuli on passive, stimulus-based tests. The finding of cortical complexity, as measured by the PCI, adds another important axis to the assessment of patients with disorders of consciousness, with sufficiently complex cortical responses indicating capacity for consciousness; however, what subjective experiences such findings might entail remain underexplored. Resting-state indices of brain function and measurements of brain metabolism via PET constitute other emerging axes of assessment to capture capacity for consciousness in the absence of overt responsiveness (Pugin et al. 2020, Candia-Rivera et al. 2021, Thibaut et al. 2021, Boerwinkle et al. 2022, Escrichs et al. 2022, Wang et al. 2022). Adding yet another layer of nuance, among those with intact passive cortical responses to sensory stimuli, some might only demonstrate unimodal sensory cortex responses, whereas others might exhibit more complex, multimodal/associative cortex responses (e.g. processing of sound versus semantics or of light versus faces). Diagnostically, the latter subsets of patients occupy a liminal space within what is already a consummate liminal space; while revealing preserved cortical processing, their cognitive function may be too impaired to facilitate discernible instruction-following with willful modulation of brain activity. What the phenomenological significance of these findings might be and whether they are constitutive of or merely concomitant with consciousness remain debated (Young et al. 2021). Does CCP demonstrate conscious perception, or is it impossible for a passive paradigm to prove that a person is conscious? (Davis et al. 2007) As this field develops, CCP may forseeably be substratified into different diagnostic subcategories reflecting the heterogeneity of potential passive cortical responses and range of endotypes, just as MCS has been substratified into MCS+, MCS (Thibaut et al. 2021) and MCS (Bruno et al. 2011). To advance precision assessment of emerging consciousness following severe brain injury, results of different modalities, including task-based fMRI/EEG/PET; resting-state fMRI/EEG/PET; stimulus-based fMRI/EEG/PET; TMS-EEG PCI, where available, may be integrated into a multimodal approach to diagnosis (Fig. 14). Multivariate results of diagnostic tests could be unified, displayed, and communicated to surrogates and clinical teams, and composite results may be used to illustrate DoC endotypes (Fig. 15).
Figure 14.
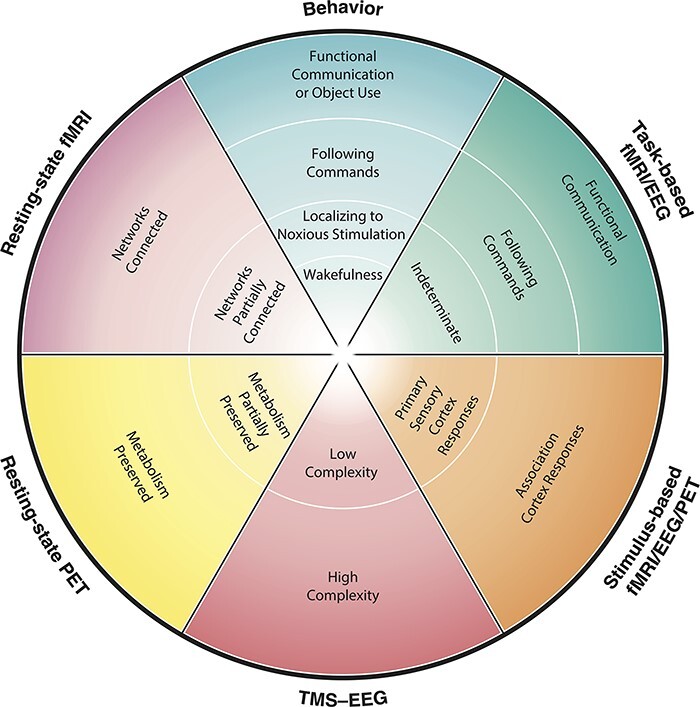
Toward precision assessment of emerging consciousness following severe brain injury. While behavior-based approaches to characterizing consciousness currently predominate in clinical settings, they are by definition insensitive to covert signs of preserved brain function which may carry diagnostic and prognostic significance. Advanced neurotechnologies now permit more precise assessment of patients’ conditions beyond overt behaviors, giving rise to new diagnostic categories including CCP in patients who might otherwise be considered to unresponsive based on behavioral testing alone. Results of different modalities, including task-based fMRI/EEG/PET; resting-state fMRI/EEG/PET; stimulus-based fMRI/EEG/PET; TMS-EEG perturbational complexity index (PCI), where available, could be integrated into a multimodal approach to advance precision assessment of emerging consciousness following severe brain injury. A radar chart such as displayed here may be used to unify, display, and communicate multivariate results of diagnostic tests to surrogates and clinical teams. The outer limit of the radar represents the most positive potential result for each diagnostic modality along a continuum of possible findings from negative (inner limit of radar) to positive (outer limit of radar). Response levels are modality-specific and positioning on the radar does not imply inter-modality equivalence with respect to the level of consciousness implied at each ring level across modalities. EEG = electroencephalography; fMRI = functional magnetic resonance imaging; PET = positron emission tomography; TMS-EEG = transcranial magnetic stimulation paired with EEG
Figure 15.
Modeling representative endotypes of disorders of consciousness (DoC) phenotypes. Each DoC is displayed with a representative constellation of multimodal findings compatible with a potential maximum composite diagnostic terminus for each condition. The outer limit of the radar represents the most positive potential result for each diagnostic modality along a continuum of possible findings from negative (inner limit of radar) to positive (outer limit of radar). While not inclusive of all combinations of possible results, potentially representative endotypes for each condition are displayed here. These examples are not intended to reflect definitive constellations, recognizing that further research is necessary to clarify optimally representative classifications with respect to phenomenology of consciousness and prognosis. Color shading represents the continuous, non-discrete nature of results. CBC = covert brain complexity; CCP = covert cortical processing; CMD = cognitive-motor dissociation; EEG = electroencephalography; eMCS = emerged from minimally conscious state; fMRI = functional magnetic resonance imaging; MCS- = minimally conscious state without command following or language function; MCS+ = minimally conscious state with command following or language function; PET = positron emission tomography; TMS-EEG = transcranial magnetic stimulation paired with electroencephalography; UWS = unresponsive wakefulness syndrome; VS = vegetative state
Directing attention outward—uncovering the phenomenology of CCP
Debate persists about whether a patient with CCP is truly consciously aware of relevant stimuli presented, or whether such findings merely track perceptual capacity and may be necessary but insufficient for phenomenal awareness. It is notable, however, that signatures of higher-level linguistic processing are absent during sleep, disrupted under conditions of distraction and inattention, and when listening to language that is not comprehended, suggesting that such findings may indeed track some degree of perceptual awareness (Makov et al. 2017, Ding et al. 2018). At the least, the finding of CCP more generally may suggest an intact ability to receive, sense and process a salient stimulus—abilities which according to some theorists may themselves be hallmarks of consciousness (Searle 1991, Graziano 2022). For example, in the case of higher-order speech processing, a person is demonstrably able not only to encode basic units of sub-lexical auditory information (i.e. phonemes), but also to potentially apply lexical knowledge to group sequences of sounds and syllables within a stream of auditory information to discern prosodic boundaries and form linguistic representations of words and higher-level syntactic structure, a process which has been recent shown to rely on intact attentional mechanisms, computational capacities, and linking to stored lexical knowledge (Braiman et al. 2018, Ding et al. 2018, Brodbeck et al. 2018, Glushko et al. 2022, Norman-Haignere et al. 2022, Gwilliams et al. 2022).
Among the most common questions family members ask physicians about their loved ones following severe brain injury is “are they in there?” or “can they hear me?”. To the extent that clinicians can no longer justifiably rely on the behavioral exam to answer these questions, the use of advanced techniques to discern depth of CCP and potentially preserved awareness is necessary to begin to formulate more reliable responses and counsel families accordingly.
Why CCP matters and paths forward
Beyond advancing scientific and philosophical understandings of consciousness, efforts to identify and characterize CCP carry important clinical implications. Given the potential diagnostic and prognostic significance of CCP, novel opportunities for neurorehabilitation may be revealed and goals-of-care discussions may be importantly informed by its discovery (Comanducci et al. 2020, Morlet et al. 2022, Naci and Owen 2022). Querying CCP among patients who lack self-expression may allow clinicians to peer beyond the often-misleading veil of behaviors to characterize and quantify residual perceptual capacities, informing family and clinical teams’ understandings of a behaviorally unresponsive patient’s condition and grounds for the possibility of conscious experience. The presence or absence of CCP could likewise guide more evidence-based resource stewardship. Greater recognition of CCP as a distinct nosologic entity could also inform clinical trials and invigorate discovery by serving as a quantifiable metric of consciousness and marker of its recovery beyond behavioral outcome measures that may be too crude to discern meaningful changes in persons who are severely brain injured and have not yet recovered overt expression (Young et al. 2022).
It is therefore imperative to sensitize prevailing diagnostic approaches to disorders of consciousness in clinical practice to the possibility of CCP so that these benefits can be captured and sustained. Much as genetic advances have transformed classification of tumor types (Louis et al. 2021) and advances in dynamic cardiac imaging (e.g. echocardiography) have transformed management approaches in cardiology, the time has come for the clinical field of disorders of consciousness care and its practitioners to revise misleading yet still prevailing diagnostic criteria that fail to capture the possibility of CCP or CMD in the realm of possible nomenclature, limited to the rough and often misleading categories of VS/UWS, MCS, MCS±, confusional state, and eMCS, despite early admonishments by Jennett and Plum that “the name for the syndrome should not imply more than is known” (Jennett and Plum 1972). 2018 and 2020 professional society guidelines for management of patients with disorders of consciousness (Giacino et al. 2018, Kondziella et al. 2020) accordingly emphasize the importance of advanced neuroimaging and electrophysiological techniques in evaluating behaviorally unresponsive patients with disorders of consciousness, but uptake of these aspirational recommendations in clinical practice has been limited (Young and Edlow 2021, Helbok et al. 2022, Monti and Schnakers 2022, Peterson et al. 2022, Young and Peterson 2022). To better reflect the current state of science, the development and adoption of revised diagnostic criteria and accompanying nosology must integrate indicators of CCP and cognitive-motor dissociation to enable more accurate and guideline-consistent approaches to classification of consciousness and its multiform disorders (Table 1) (Young and Edlow 2021, Peterson et al. 2022, Young and Peterson 2022). To facilitate improved tracking and reporting, new ICD codes are necessary to capture CCP and CMD, and outdated diagnostic approaches that hinge solely on behavioral criteria must be accordingly jettisoned or appropriately contextualized.
Table 1.
Proposed taxonomy of behaviorally unresponsive disorders of consciousness (DoC): salient conceptual, clinical, and research considerations.
| Taxonomy | Clinical concept of consciousness | Exemplative neurotechnological indicator(s) | Clinical implications | Research implications |
|---|---|---|---|---|
| Covert cortical processing (CCP) | Intact association cortex responses to stimuli in the absence of behavioral evidence of stimulus processing (e.g. language comprehension) or command-following. | Stimulus-based fMRI response Stimulus-based EEG response |
Patients diagnosed with coma, vegetative state/unresponsive wakefulness syndrome (VS/UWS), or low-level minimally conscious state (MCS-) by behavioral criteria could exhibit signs of association cortex responsiveness on stimulus-based testing that exceed what is implied by a behavioral diagnosis. Outcomes (prognosis) and capacity for experience (phenomenology) may differ from those who lack such responses. Prevailing behavior-based approaches to clinical diagnosis of DoC are inherently insensitive to CCP and should be interpreted accordingly. To enhance the reliability of clinical DoC assessment, CCP should be queried when possible, and surrogate decision-makers should be counseled about what is known and unknown about CCP. |
Underexplored opportunities exist for further empirical studies of phenomenology and prognosis in CCP. Studies of unresponsive patients with DoC that do not include measures of CCP risk erroneous miscategorization or conflation of heterogeneous subpopulations. |
| Cognitive-motor dissociation (CMD)/covert consciousness | Intact volitional modulation of brain activity, despite absence of language function on routine bedside behavioral evaluation (Schiff 2015) | Task-based fMRI response Task-based EEG response |
Patients who fail to respond to routine behavioral testing at the bedside may still harbor capacities to volitionally modulate brain activity or consciously respond only through advanced testing. Prevailing behavior-based approaches to clinical diagnosis and prognosis of DoC are inherently insensitive to CMD and should be interpreted accordingly. Patients diagnosed with low-level MCS (MCS-), VS or coma by prevailing behavioral criteria may exhibit signs of awareness that exceed their behavioral diagnosis. Generally more favorable prognosis for functional recovery as compared to behaviorally unresponsive patients without CMD (Egbebike et al. 2022, Claassen et al. 2019) |
Further empirical studies of phenomenology, mechanisms, and correlates of CMD are needed. Studies of unresponsive patients with DoC that do not include measures of CMD risk erroneous miscategorization or conflation of heterogeneous subpopulations, including mislabeling patients who are aware and covertly responsive as unconscious. |
| Covert brain complexity (CBC) | Intact ability of a patient’s brain to sustain complex dynamics resembling those seen in conscious states despite a behavioral diagnosis of coma or VS/UWS, and absence of discernible responses to sensory-based stimuli. | Transcranial Magnetic Stimulation—Electroencephalography Perturbational Complexity Index (TMS-EEG PCI) | Some patients may demonstrate patterns of preserved brain complexity (i.e. high PCI value in response to TMS perturbation) but due to sensory, motor and/or cognitive impairments exhibit no other covert or overt signs of consciousness. In patients with known sensory, motor or cognitive impairments, tests that bypass afferent and efferent pathways and causally probe network connectivity implicated in human consciousness may prove more reliable (Edlow et al. 2023) |
Further studies comparing diagnostic and prognostic value of complexity measures with a composite reference standard including behavioral, task-based, stimulus-based and resting-state measures are needed, and may help to illuminate optimal approaches to DoC classification and prediction. Whether the brain’s capacity to sustain complex dynamics as indexed by PCI is a necessary condition for CCP has yet to be empirically studied, but is a theoretically plausible hypothesis. |
Open questions and opportunities
Several outstanding questions problematize the discovery and diagnosis of CCP in clinical practice. The phenomenological significance of CCP, specifically, what it is like to be in a state of CCP, is currently unknown. Efforts to investigate this are underway, but further studies are crucially needed to provide a window into the subjective experience of those in this condition and to shed light on quality of life and on interventions that might support wellbeing. Opportunities to study differences and similarities between CCP in states of disordered versus non-disordered consciousness have additionally been underexplored but are prime grounds for potentially fruitful discovery; for example, how might forms of seemingly unaware, subconscious, or subliminal cognitive processing in healthy states inform approaches to understanding CCP in patients with brain injury, or vice versa (Dehaene et al. 2006, Naccache 2018)? Could studying possible forms of CCP in other states, such as unconscious knowing (justified true beliefs held without awareness), unconscious processing (priming; subconscious perception); or unconscious emotional states (e.g. repression; suppression; dissociation), potentially yield new ways to diagnose and characterize CCP in states of disordered consciousness? How might recent discoveries pertaining to CCP potentially clarify philosophical controversies about consciousness, intentionality, attention, and perception? Additionally, given the diagnostic grey-areas that exist, especially when considering conditions such as CMD, how can we be confident that a patient characterized as CCP is not also experiencing CMD in cases where task-based fMRI results are negative, in light of the imperfect sensitivity of such tests? Is CCP a graded or all-or-none condition? Approaches that combine different neuroimaging and neurophysiological measures are needed to improve the reliability of differentiating between such states, but in the absence of a true “gold-standard” assessment of consciousness outside of awareness in the first-person instance (Velleman 1996) some uncertainty is likely to remain. Finally, dedicated studies on tailored therapeutic and prognostic approaches for CCP are needed to improve management strategies across the disorders of consciousness care continuum.
Reimagining the clinical landscape of consciousness—conclusions
CCP is an emerging and clinically relevant state of consciousness marked by the presence of intact association cortex responses to environmental stimuli in the absence of behavioral evidence of stimulus processing. CCP is not a monotonic state but rather encapsulates a spectrum of possible association cortex responses from rudimentary to complex, and to a range of possible passive stimuli. Despite decades of progress, the prevailing nosology used by clinicians worldwide to classify patients with disorders of consciousness remains dangerously fixed in the antiquated behaviorist orthodoxy that perniciously equates lack of motoric expression with lack of awareness.
Educational efforts to inform clinicians and researchers of this condition are crucial, along with efforts to sensitize diagnostic criteria and disorders of consciousness nosology to these vital discoveries, and to democratize access to the resources necessary for clinical identification of CCP. As Wittgenstein cautioned, it is often the case that “we want to understand something that is already in plain view…[many] problems are solved not by seeking new information, but by arranging what we have long since known” (Wittgenstein, 1953). Opportunities for further study of CCP are immense, and research teams with multidisciplinary expertise including translational neuroscience, neurology, philosophy, ethics, and implementation science are particularly well positioned to advance and organize knowledge in this field to improve the care of patients with these conditions around the world.
Acknowledgements
This work was supported by the NIH BRAIN Initiative (F32MH123001), NIH National Institute of Neurological Disorders and Stroke (R21NS109627 and RF1NS115268), NIH Director’s Office (DP2HD101400), Henry and Allison McCance Center for Brain Health/Mass General Neuroscience SPARC Award, James S. McDonnell Foundation, Tiny Blue Dot Foundation, Chen Institute MGH Research Scholar Award, and Mass General Neuroscience Transformative Scholar Award. We are deeply grateful for the insightful reviews of the three anonymous reviewers. We thank Kimberly Main Knoper for enhancing the artwork for Figs 14 and 15.
Contributor Information
Michael J Young, Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, 101 Merrimac Street, Suite 310, Boston, MA 02114, USA.
Matteo Fecchio, Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, 101 Merrimac Street, Suite 310, Boston, MA 02114, USA.
Yelena G Bodien, Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, 101 Merrimac Street, Suite 310, Boston, MA 02114, USA; Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, 300 1st Ave, Charlestown, Boston, MA 02129, USA.
Brian L Edlow, Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, 101 Merrimac Street, Suite 310, Boston, MA 02114, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, 149 13th St, Charlestown, Charlestown, MA 02129, USA.
Conflict of interest
None declared.
Data availability
No new data were created.
References
- Andrews K, Murphy L, Munday R et al. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. Bmj 1996;313:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio MK, Christos L ‘Conceptualizing Consciousness: a Change in Perspective: The Elephant Still Surprises Those only Touching Its Trunk’. Physical Medicine and Rehabilitation Clinics 2023;35:1–13. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Pistoia F, Sarà M et al. Resting state eyes-closed cortical rhythms in patients with locked-in-syndrome: an EEG study. Clin Neurophys 2010;121:1816–24. [DOI] [PubMed] [Google Scholar]
- Bayne T, Hohwy J, Owen AM. Response to ‘Minimally conscious state or cortically mediated state?’. Brain 2018;141:e26–e26. [DOI] [PubMed] [Google Scholar]
- Berkovitch L, Charles L, Del Cul A et al. Disruption of conscious access in psychosis is associated with altered structural brain connectivity. J Neurosci 2021;41:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlad I, Pratt H. P300 in response to the subject’s own name. Electroencephalogr Clin Neurophysiol /Evoked Potentials Section 1995;96:472–4. [DOI] [PubMed] [Google Scholar]
- Bernat JL. Questions remaining about the minimally conscious state. Neurology 2002;58:337–8. [DOI] [PubMed] [Google Scholar]
- Beukema S, Gonzalez-Lara LE, Finoia P et al. A hierarchy of event-related potential markers of auditory processing in disorders of consciousness. NeuroImage 2016;12:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Rockwood K. Delirium as a disorder of consciousness. J Neurol Neurosurg Psychiatry 2007;78:1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boegle K, Bassi M, Comanducci A et al. Informal caregivers of patients with disorders of consciousness: a qualitative study of communication experiences and information needs with physicians. Neuroethics 2022;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerwinkle VL, Sussman BL, Manjón I et al. Association of network connectivity via resting state functional MRI with consciousness, mortality, and outcomes in neonatal acute brain injury. NeuroImage 2022;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C, Nydam AS, Dux PE et al. State-dependent effects of neural stimulation on brain function and cognition. Nat Rev Neurosci 2022;23:459–75. [DOI] [PubMed] [Google Scholar]
- Braiman C, Fridman EA, Conte MM et al. Cortical response to the natural speech envelope correlates with neuroimaging evidence of cognition in severe brain injury. Curr Biol 2018;28:3833–3839. e3. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Rektor I, Daniel P et al. Intracerebral event-related potentials to subthreshold target stimuli. Clin Neurophysiol 2001;112:650–61. [DOI] [PubMed] [Google Scholar]
- Brodbeck C, Hong LE, Simon JZ. Rapid transformation from auditory to linguistic representations of continuous speech. Curr Biol 2018;28:3976–3983. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M-A, Vanhaudenhuyse A, Thibaut A et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011;258:1373–84. [DOI] [PubMed] [Google Scholar]
- Candia-Rivera D, Annen J, Gosseries O et al. Neural responses to heartbeats detect residual signs of consciousness during resting state in postcomatose patients. J Neurosci 2021;41:5251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll EE, Der-Nigoghossian C, Alkhachroum A et al. Common data elements for disorders of consciousness: recommendations from the electrophysiology working group. Neurocrit Care 2023;39:578–585. [DOI] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5:198ra105–198ra105. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016;80:718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, Bragdon HR. Evoked responses to numerical and non-numerical visual stimuli while problem solving. Nature 1964;203:1155–7. [DOI] [PubMed] [Google Scholar]
- Choi W, Young MJ. Disambiguating Consciousness in Clinical Settings. Neurology 2023;101:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J, Doyle K, Matory A et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380:2497–505. [DOI] [PubMed] [Google Scholar]
- Claassen J, Kondziella D, Alkhachroum A et al. Cognitive motor dissociation: gap analysis and future directions. Neurocrit Care 2023:1–18. [DOI] [PubMed] [Google Scholar]
- Coleman M, Bekinschtein T, Monti M et al. A multimodal approach to the assessment of patients with disorders of consciousness. Prog Brain Res 2009a;177:231–48. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Davis MH, Rodd JM et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009b;132:2541–52. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Rodd JM, Davis MH et al. Do vegetative patients retain aspects of language comprehension? Evidence from fMRI. Brain 2007;130:2494–507. [DOI] [PubMed] [Google Scholar]
- Colombo MA, Comanducci A, Casarotto S et al. Beyond alpha power: EEG spatial and spectral gradients robustly stratify disorders of consciousness. Cereb Cortex 2023;33:7193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comanducci A, Boly M, Claassen J et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group. Clin Neurophys 2020;131:2736–65. [DOI] [PubMed] [Google Scholar]
- Comanducci A, Casarotto S, Rosanova M et al. Unconsciousness or unresponsiveness in akinetic mutism? Insights from a multimodal longitudinal exploration. Eur J Neurosci 2023:1–14. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2011;378:2088–94. [DOI] [PubMed] [Google Scholar]
- Daltrozzo J, Wioland N, Mutschler V et al. Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis. Clin Neurophysiol 2007;118:606–14. [DOI] [PubMed] [Google Scholar]
- Daly I. Neural decoding of music from the EEG. Sci Rep 2023;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci 2007;104:16032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux J-P, Naccache L et al. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 2006;10:204–11. [DOI] [PubMed] [Google Scholar]
- de Jong BM, Willemsen A, Paans A. Regional cerebral blood flow changes related to affective speech presentation in persistent vegetative state. Clin Neurol Neurosurg 1997;99:213–6. [DOI] [PubMed] [Google Scholar]
- Del Pin SH, Skóra Z, Sandberg K et al. Comparing theories of consciousness: why it matters and how to do it. Neurosci Conscious 2021;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Pan X, Luo C et al. Attention is required for knowledge-based sequential grouping: insights from the integration of syllables into words. J Neurosci 2018;38:1178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di H, Yu S, Weng X et al. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology 2007;68:895–9. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Chatelle C, Spencer CA et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140:2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Claassen J, Schiff ND et al. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol 2021;17:135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Fecchio M, Bodien YG et al. Measuring consciousness in the intensive care unit. Neurocrit Care 2023;38:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Naccache L. Unmasking covert language processing in the intensive care unit with electroencephalography. Ann Neurol 2021;89:643–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbebike J, Shen Q, Doyle K et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol 2022;21:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrichs A, Perl YS, Uribe C et al. Unifying turbulent dynamics framework distinguishes different brain states. Commun Biol 2022;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MJ, Nielsen TA, Paquette T. Reduced Alpha power associated with the recall of mentation from Stage 2 and Stage REM sleep. Psychophysiology 2004;41:288–97. [DOI] [PubMed] [Google Scholar]
- European Delirium Association and American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Medicine 2014;12:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espejo D, Rossit S, Owen AM. A thalamocortical mechanism for the absence of overt motor behavior in covertly aware patients. JAMA Neurol 2015;72:1442–50. [DOI] [PubMed] [Google Scholar]
- Fins JJ. Rights Come to Mind: Brain Injury, Ethics, and the Struggle for Consciousness. New York, NY: Cambridge University Press, 2015. [Google Scholar]
- Frohlich J, Toker D, Monti MM. Consciousness among delta waves: a paradox?. Brain 2021;144:2257–77. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–9. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Katz DI, Schiff ND et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018;91:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino J, Zasler N, Whyte J et al. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil 1995;76:205–9. [DOI] [PubMed] [Google Scholar]
- Giraud A-L, Su Y. Reconstructing language from brain signals and deconstructing adversarial thought-reading. Cell Rep Med 2023;4:101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushko A, Poeppel D, Steinhauer K. Overt and implicit prosody contribute to neurophysiological responses previously attributed to grammatical processing. Sci Rep 2022;12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden K, Bodien YG, Giacino JT. Disorders of Consciousness: Classification and Taxonomy. Phys Med Rehabil Clin 2023;35:15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AM, Victor JD, Conte MM et al. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophys 2011;122:2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. A conceptual framework for consciousness. Proc Natl Acad Sci 2022;119:e2116933119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui P, Jiang Y, Zang D et al. Assessing the depth of language processing in patients with disorders of consciousness. Nat Neurosci 2020;23:761–70. [DOI] [PubMed] [Google Scholar]
- Gwilliams L, King J-R, Marantz A et al. Neural dynamics of phoneme sequences reveal position-invariant code for content and order. Nat Commun 2022;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker P. Wittgenstein’s Legacy: The Principles of the Private Language Arguments. Philos Investig 2018;41:123–40. [Google Scholar]
- Heiss W-D, Gerstenbrand F, Prosenz P et al. The prognostic value of cerebral blood flow measurement in patients with the apallic syndrome. J Neurol Sci 1972;16:373–82. [DOI] [PubMed] [Google Scholar]
- Helbok R, Rass V, Beghi E et al. The curing coma campaign international survey on coma epidemiology, evaluation, and therapy (COME TOGETHER). Neurocrit Care 2022;37:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Sangaré A, Munoz-Musat E et al. Importance, limits and caveats of the use of “disorders of consciousness” to theorize consciousness. Neurosci Conscious 2021;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH, Ciria MG Assessment of severe damage to the brain by multiregional measurements of cerebral blood flow. In Symposium on the Outcome of Severe Damage to the Central Nervous System held at the Ciba Foundation, London, on 26-28th November, 1974, 97–120. Wiley Online Library, 1975. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Schwartz MS. Blood flow patterns induced in the dominant hemisphere by speech and reading. Brain 1974;97:273–88. [DOI] [PubMed] [Google Scholar]
- Jennett B, Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet 1972;299:734–7. [DOI] [PubMed] [Google Scholar]
- Kety SS. Circulation and metabolism of the human brain in health and disease. Am J Med 1950;8:205–17. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Investig 1948;27:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee U, Vlisides PE. Delirium and cortical complexity. J Gerontol A 2022;77:2219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella D, Bender A, Diserens K et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol 2020;27:741–56. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Menon DK, Helbok R et al. A precision medicine framework for classifying patients with disorders of consciousness: Advanced Classification of Consciousness Endotypes (ACCESS). Neurocrit Care 2021;35:27–36. [DOI] [PubMed] [Google Scholar]
- Kronemer SI, Aksen M, Ding JZ et al. Human visual consciousness involves large scale cortical and subcortical networks independent of task report and eye movement activity. Nat Commun 2022;13:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Faymonville M-E, Degueldre C et al. Auditory processing in the vegetative state. Brain 2000;123:1589–601. [DOI] [PubMed] [Google Scholar]
- Laureys S, Faymonville M-E, Peigneux P et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002;17:732–41. [PubMed] [Google Scholar]
- Lazaridis C. Withdrawal of life-sustaining treatments in perceived devastating brain injury: the key role of uncertainty. Neurocrit Care 2019;30:33–41. [DOI] [PubMed] [Google Scholar]
- Lee M, Sanz LR, Barra A et al. Quantifying arousal and awareness in altered states of consciousness using interpretable deep learning. Nat Commun 2022;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Sidtis JJ, Rottenberg DA et al. Differences in cerebral blood flow and glucose utilization in vegetative versus locked‐in patients. Ann Neurol 1987;22:673–82. [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Wesseling P et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology 2021;23:1231–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makov S, Sharon O, Ding N et al. Sleep disrupts high-level speech parsing despite significant basic auditory processing. J Neurosci 2017;37:7772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCurdy JT. Problems in Dynamic Psychology. J Neurosci, New York: Macmillan Publishers, 1922. [Google Scholar]
- Mediano PA, Rosas FE, Bor D et al. The strength of weak integrated information theory. Trends Cogn Sci 2022;26:646–55. [DOI] [PubMed] [Google Scholar]
- Menon DK, Owen A, Boniface S et al. Cortical processing in persistent vegetative state. Lancet 1998;352:1148–9. [DOI] [PubMed] [Google Scholar]
- Monti MM, Schnakers C. Flowchart for Implementing Advanced Imaging and Electrophysiology in Patients With Disorders of Consciousness: To fMRI or Not to fMRI?. Neurology 2022;98:452–9. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–89. [DOI] [PubMed] [Google Scholar]
- Morlet D, Mattout J, Fischer C et al. Infraclinical detection of voluntary attention in coma and post-coma patients using electrophysiology. Clin Neurophys 2022;145:151–61. [DOI] [PubMed] [Google Scholar]
- Naccache L. Minimally conscious state or cortically mediated state?. Brain 2018;141:949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L. Reply: response to ‘Minimally conscious state or cortically mediated state?’. Brain 2018;141:e27–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L. Why and how access consciousness can account for phenomenal consciousness. Philos Trans R Soc B 2018;373:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci L, Owen AM. Uncovering consciousness and revealing the preservation of mental life in unresponsive brain-injured patients. Seminars in Neurology 2022;42:299–308. [DOI] [PubMed] [Google Scholar]
- Nemirovsky IE, Popiel NJ, Rudas J et al. An implementation of integrated information theory in resting-state fMRI. Commun Biol 2023;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Haignere SV, Long LK, Devinsky O et al. Multiscale temporal integration organizes hierarchical computation in human auditory cortex. Nat Hum Behav 2022;6:455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura Y, Asano Y, Takenaka S et al. Brain activation by music in patients in a vegetative or minimally conscious state following diffuse brain injury. Brain Injury 2014;28:944–50. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M et al. Detecting awareness in the vegetative state. Science 2006;313:1402–1402. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Menon DK et al. Using a hierarchical approach to investigate residual auditory cognition in persistent vegetative state. Prog Brain Res 2005;150:457–608. [DOI] [PubMed] [Google Scholar]
- Ozcelik F, VanRullen R. Natural scene reconstruction from fMRI signals using generative latent diffusion. Sci Rep 2023;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Schnakers C, Schabus M et al. Brain response to one’s own name in vegetative state, minimally conscious state, and locked-in syndrome. Archives of neurology 2006;63:562–9. [DOI] [PubMed] [Google Scholar]
- Peterson A, Young MJ, Fins JJ. Ethics and the 2018 practice guideline on disorders of consciousness: a framework for responsible implementation. Neurology 2022;98:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum F, Posner JB. The Diagnosis of Stupor and Coma. USA: Oxford University Press, 1982. [Google Scholar]
- Pugin D, Hofmeister J, Gasche Y et al. Resting-state brain activity for early prediction outcome in postanoxic patients in a coma with indeterminate clinical prognosis. Am J Neuroradiol 2020;41:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PVS Multisociety Task Force . Medical aspects of the persistent vegetative state. N Engl J Med 1994;330:1499–508. [DOI] [PubMed] [Google Scholar]
- Ribary U, Schiff N, Kronberg E et al. Fractured brain function in unconscious humans II: Functional brain imaging using MEG. NeuroImage 1998;7:S106. [Google Scholar]
- Risberg J, Ingvar DH. Multibolus technique for measuring the distribution of cerebral blood flow over short intervals in man. Circ Res 1972;31:889–98. [DOI] [PubMed] [Google Scholar]
- Risberg J, Ingvar DH. Patterns of activation in the grey matter of the dominant hemisphere during memorizing and reasoning: a study of regional cerebral blood flow changes during psychlogical testing in a group of neurologically normal patients. Brain 1973;96:737–56. [DOI] [PubMed] [Google Scholar]
- Rorty R. Incorrigibility as the mark of the mental. J Philo 1970;67:399–424. [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1890;11:85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, Boly M, Napolitani M et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr Biol 2015;25:3099–105. [DOI] [PubMed] [Google Scholar]
- Sarasso S, Casali AG, Casarotto S et al. Consciousness and complexity: a consilience of evidence. Neurosci Conscious 2021;7:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72:1413–5. [DOI] [PubMed] [Google Scholar]
- Schmidt CF. The influence of cerebral blood-flow on respiration: I. The respiratory responses to changes in cerebral blood-flow. Am J PhysiolLegacy Content 1928a;84:202–22. [Google Scholar]
- Schmidt CF. The influence of cerebral blood-flow on respiration: II. The gaseous metabolism of the brain. Am J PhysiolLegacy Content 1928b;84:223–41. [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopenhauer A The world as will and representation (EFJ Payne, Trans.). NY: Dover, 1969. [Google Scholar]
- Scotti PS, Banerjee A, Goode J et al. Reconstructing the Mind’s Eye: fMRI-to-Image with Contrastive Learning and Diffusion Priors. Presented at 37th Conference on Neural Information Processing Systems (NeurIPS 2023) in New Orleans, LA USA. pp. 1–24. [Google Scholar]
- Searle JR. Consciousness, unconsciousness and intentionality. Philos Issues 1991;1:45–66. [Google Scholar]
- Seth AK, Bayne T. Theories of consciousness. Nat Rev Neurosci 2022;23:439–52. [DOI] [PubMed] [Google Scholar]
- Shalit M, Beller A, Feinsod M. Clinical equivalents of cerebral oxygen consumption in coma. Neurology 1972;22:155–155. [DOI] [PubMed] [Google Scholar]
- Shalit M, Beller A, Feinsod M et al. The blood flow and oxygen consumption of the dying brain. Neurology 1970;20:740–740. [DOI] [PubMed] [Google Scholar]
- Shapiro-Rosenbaum A, Jaffe MP. Education, training, and support across the continuum of recovery for caregivers of persons with disorders of consciousness. Phys Med Rehabil Clin 2023;35:193–208. [DOI] [PubMed] [Google Scholar]
- Sherer M, Katz DI, Bodien YG et al. Post-traumatic confusional state: a case definition and diagnostic criteria. Arch Phys Med Rehabil 2020;101:2041–50. [DOI] [PubMed] [Google Scholar]
- Sokoliuk R, Degano G, Banellis L et al. Covert speech comprehension predicts recovery from acute unresponsive states. Ann Neurol 2021;89:646–56. [DOI] [PubMed] [Google Scholar]
- Sorger B, Goebel R. Real-time fMRI for brain-computer interfacing. Handb Clin Neurol 2020;168:289–302. [DOI] [PubMed] [Google Scholar]
- Stender J, Gosseries O, Bruno M-A et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014;384:514–22. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Parker M, Lennertz R et al. Delirium and cortical complexity: divergent changes in alpha and theta bands. J Gerontol A 2022;77:2221–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, LeBel A, Jain S et al. Semantic reconstruction of continuous language from non-invasive brain recordings. Nat Neurosci 2023;26:858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut A, Panda R, Annen J et al. Preservation of brain activity in unresponsive patients identifies MCS star. Ann Neurol 2021;90:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Consciousness and complexity. science 1998;282:1846–51. [DOI] [PubMed] [Google Scholar]
- Velleman JD. Self to self. Philos Rev 1996;105:39–76. [Google Scholar]
- Walter N, Hinterberger T. Determining states of consciousness in the electroencephalogram based on spectral, complexity, and criticality features. Neurosci Conscious 2022;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Di H, Hu X et al. Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Medicine 2015;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Ma X et al. Regional Homogeneity Alterations in Patients with Impaired Consciousness. An Observational Resting-State fMRI Study. Neuroradiology 2022;64:1391–9. [DOI] [PubMed] [Google Scholar]
- Whiteley CM. Aphantasia, imagination and dreaming. Philos Stud 2021a;178:2111–32. [Google Scholar]
- Whiteley CM. Depression as a disorder of consciousness. Br J Philos Sci 2021b. [Google Scholar]
- Wickens C, Kramer A, Vanasse L et al. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science 1983;221:1080–2. [DOI] [PubMed] [Google Scholar]
- Willett FR, Kunz EM, Fan C et al. A high-performance speech neuroprosthesis. Nature 2023;620:1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H, Golbabaee M, Proulx MJ et al. EEG-based BCI dataset of semantic concepts for imagination and perception tasks. Sci Data 2023;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittgenstein L. Philosophical Investigations. Oxford: Blackwell Publishers, 1953. [Google Scholar]
- Young MJ. Pathologies of thought and first-person authority. Philosophy Psychiatry Psychol 2018;25:151–9. [Google Scholar]
- Young MJ. Disorders of Consciousness Rehabilitation: Ethical Dimensions and Epistemic Dilemmas. Physical Medicine and Rehabilitation Clinics 2023;35:209–21. [DOI] [PubMed] [Google Scholar]
- Young MJ, Bodien YG, Edlow BL. Ethical considerations in clinical trials for disorders of consciousness. Brain Sci 2022;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Bodien YG, Giacino JT et al. The neuroethics of disorders of consciousness: a brief history of evolving ideas. Brain 2021;144:3291–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Edlow BL. The quest for covert consciousness: Bringing neuroethics to the bedside. Neurology 2021;96:893–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Peterson A Neuroethics across the disorders of consciousness care continuum. Seminars in Neurology 2022;42:375–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created.