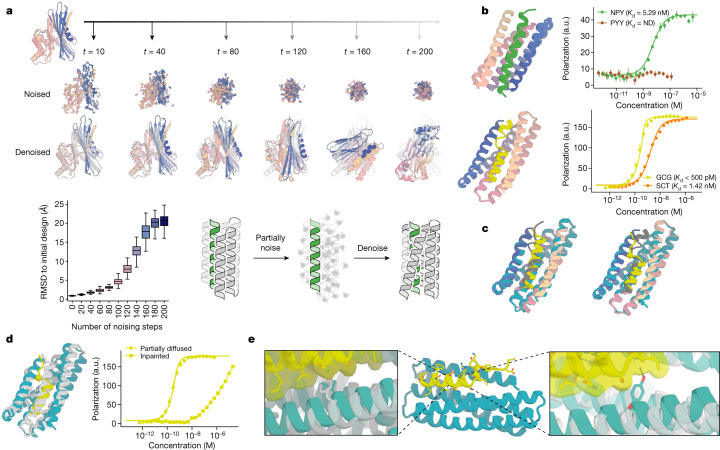
Fig. 2. Peptide binder optimization with RFdiffusion.
a, Top: partial diffusion. RFdiffusion is used to denoise a randomly noised starting design (left); varying the extent of initial noising (middle row) enables control over the extent of introduced structural variation (bottom row; colours, new designs; grey, original design). Bottom left: partial diffusion diversifies designs. Note that the greater the amount of noise added, the more dissimilar the outputs are to the starting structure. Bottom right: depiction of the helix binder optimization strategy. b, Top: design model (spectrum) of the partially diffused binder to NPY (green) and FP measurements (n = 4) indicating a 5.3 nM binding affinity to NPY target and selectivity over PYY (brown). ND, not detectable. Bottom: design model (spectrum) of the partially diffused binder to GCG (yellow) and FP measurements (n = 4) indicating a subnanomolar binding affinity to GCG and selectivity over SCT (orange). c, Left: model (spectrum with GCG in grey) aligns with 0.72 Å RMSD to the 1.95-Å crystal structure (teal and yellow) of the RFjoint Inpainted GCG binder. Right: model (spectrum with GCG in grey) aligns with 0.6 Å RMSD to the 1.81-Å crystal structure (teal and yellow) of the partially diffused GCG binder. d, Left: the crystal structures of the Inpainted (grey) and partially diffused (teal and yellow) GCG binders have considerable topological similarity; there are many small readjustments. Right: FP titrations (n = 4) with GCG indicate much tighter binding following partial diffusion. e, Left inset: the crystal structure of the partially diffused backbone (teal) shows how the newly introduced Ile13 increases shape complementarity compared to the phenylalanine in the Inpainted binder (crystal structure in grey; structures aligned on residues 16–29 of GCG). Middle: crystal structure of the partially diffused GCG binder (teal and yellow). Right inset: the backbone shifts in the partially diffused structure (teal) enable Tyr16 to make packing and hydrogen-bonding interactions with the peptide; Ser16 in the original design did not make any peptide contacts (grey).