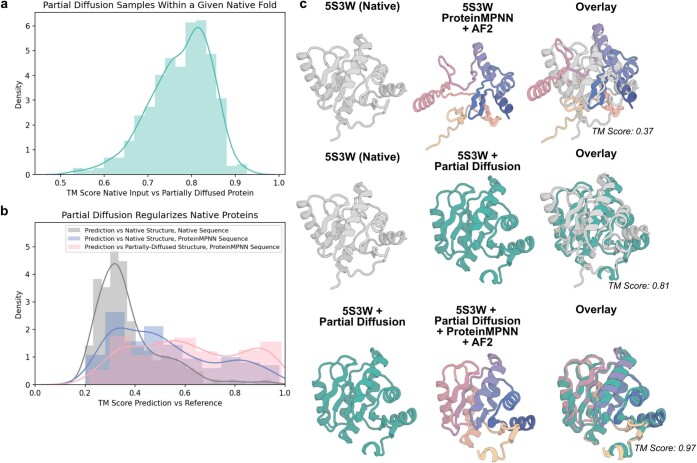
Extended Data Fig. 4. Partial diffusion increases designability of native proteins.
500 native proteins of length 100 to 300 residues were selected from the PDB (< 3.5 Å resolution and no missing residues). Three different methods were applied to these proteins: 1) single sequence AlphaFold2 (AF2), 2) ProteinMPNN combined with AF2, and 3) partial diffusion (60 steps, noise = 1), ProteinMPNN and AF2. (a) Partial diffusion generates diverse protein conformations from the initial fold while maintaining the same overall fold, as indicated by the TM (Template Modeling) score exceeding 0.5. (b) The backbones resulting from partial diffusion exhibit higher designability compared to the native backbone, implying that they have been idealised for design purposes. (c) Visualisation of an example where partial diffusion + ProteinMPNN results in a significantly more designable protein relative to sequence redesign by ProteinMPNN on the native backbone.