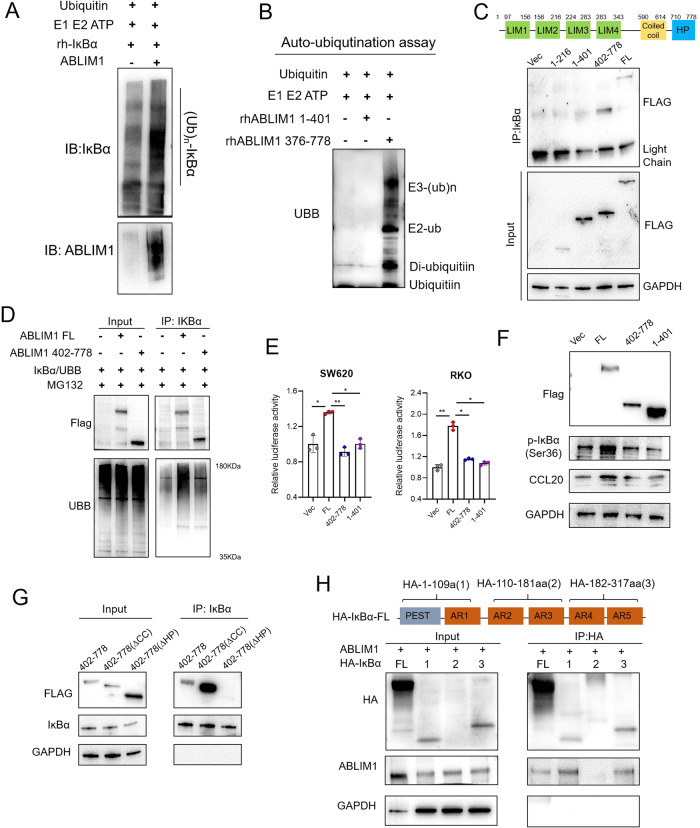
Fig. 7. ABLIM1 is a novel E3 ligase, which interacts with IκBα via HP domain and ubiquitinates it depending on 402-778aa.
A E3 ligase activity of ABLIM1 was determined by in vitro ubiquitination assay. HCT116 cells overexpressed with ABLIM1 or vector underwent IP using ABLIM1 antibody and magnetic beads, and then the beads were incubated with E1, E2, ATP, and recombinant IκBα, followed by immunoblotting examination. B Auto-ubiquitination assay revealed that recombinant ABLIM1 376-778aa rather than 1-401aa harbored E3 ligase activity. Ubiquitinated protein was detected by immunoblotting with an anti-ubiquitin antibody. C 402-778aa region of ABLIM1 was required for the interaction between ABLIM1 and IκBα. A diagram shows the structures of ABLIM1 (top). HCT116 cells were transfected with Flag-ABLIM1 constructs (full length, 1-216aa, 1-401aa, 402-778aa) or empty vector, respectively. D Co-IP assay revealed LIM domains were required for the ubiquitination activity of ABLIM1. E NF-κB transcriptional activities in RKO and SW620 cells were detected by dual-luciferase assay after transfection of different ABLIM1 mutant constructs (full length, 1-401aa, 402-778aa). n = 3 for each group. Kruskal–Wallis test: *P < 0.05; **P < 0.01. F Immunoblotting images of Flag, p-IκBα, CCL20, and GAPDH in HCT116 cells after transfection of different ABLIM1 mutant constructs. G In RKO, co-IP assay using ABLIM1 402-778aa mutant constructs (coiled-coil deleted, HP domain deleted) confirmed HP domain was responsible for the interaction between ABLIM1 and IκBα. H Western blot images of co-IPs performed on lysates of HCT116 cells transfected with ABLIM1 and indicated HA-IκBα constructs.