Abstract
Background
Cancer therapy-related cardiovascular toxicity (CTR-CVT) from immune checkpoint inhibitor (ICI) therapy is still incompletely characterized, and patients with pre-existing cardiovascular disease represent a particularly high-risk cohort. Valid parameters for risk stratification of these patients are missing. Neutrophil-to-lymphocyte ratio (NLR) has been shown to predict mortality and adverse events in other cardiovascular cohorts. The present study aims to examine the predictive capacity of NLR for risk stratification of patients particularly vulnerable for CTR-CVT under ICI therapy.
Methods
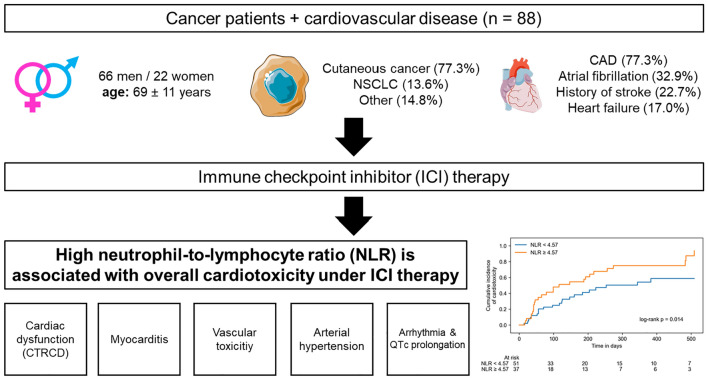
We performed an analysis of 88 cancer patients (69 ± 11 years, 25% female) with pre-existing cardiovascular disease under ICI therapy from the prospective Essen Cardio-Oncology Registry (ECoR). NLR was assessed at patient enrollment and the population was divided through receiver operator characteristic (ROC) curve analysis in patients with low (< 4.57) and high (≥ 4.57) NLR. Endpoint was the whole spectrum of CTR-CVT, according to the European guidelines on cardio-oncology. The median follow-up was 357 days (interquartile range (IQR): 150–509 days).
Results
We observed 4 cases of myocarditis, 17 cases of vascular toxicity, 3 cases of arterial hypertension, 22 cases of arrhythmia or QTc prolongation and 17 cases of cardiovascular dysfunction. NLR was associated with overall CTR-CVT by univariable Cox regression (hazard ratio (HR): 1.443; 95% confidence interval (CI) 1.082–1.925; p = 0.013). However, this association was attenuated after adjusting for further confounders.
Conclusion
NLR is moderately associated with CTR-CVT in cancer patients with pre-existing cardiovascular disease under ICI therapy. Surveillance of NLR during ICI therapy might be an effective and economically biomarker for risk stratification in these high-risk patients.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-023-02327-9.
Keywords: Immune checkpoint inhibitor, Cancer therapy-related cardiovascular toxicity, Neutrophil-to-lymphocyte ratio, Cardiovascular disease
Introduction
Survival of cancer patients has significantly improved over the last decade due to introduction of immune checkpoint inhibitor (ICI) therapy [1–6]. Among others, these treatments are used in the therapy of skin cancer and non-small-cell lung cancer [7–11]. Therapy targets are receptors on the T cell membrane and their ligands on the tumor surface, which allow the cancer cells to be falsely recognized as self-structures and enable them to evade the immune system [12, 13]. ICI therapy blocks this interaction and facilitates their elimination by immune cells [13]. Important immune checkpoint classes approved for ICI therapy so far are programmed cell death protein 1 (PD-1), programmed cell death 1 ligand-1 (PDL-1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4). PD-1 is targeted by nivolumab, pembrolizumab, cemiplimab and spartalizumab [13]. Avelumab, durvalumab and atezolizumab inhibit binding of T cells to PDL-1 on the tumor surface and ipilimumab, among others, targets cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) [13].
These therapies come along with cardiotoxic side effects in treated patients [14–16]. These treatment-related effects gained more significance due to an extension of approval in many more cancer entities with tremendous beneficial impact on morbidity and quality of life [15]. The 2022 European guidelines on cardio-oncology and the International cardio-oncology society (IC-OS) recently defined several entities of cancer therapy-related cardiovascular toxicity (CTR-CVT) [17, 18]. One aspect is cancer therapy-related cardiovascular dysfunction (CTRCD), ranging from an increase in cardiac biomarkers or decline in left ventricular contractility to decompensated heart failure. Patients can develop myocarditis or vascular toxicity, appearing as coronary, peripheral and cerebral artery disease or thromboembolic events like myocardial infarction or stroke. Further aspects are new onset of arterial hypertension and QTc prolongation as well as atrial and ventricular arrhythmia. Especially patients with pre-existing cardiovascular disease are endangered to develop CTR-CVT under ICI therapy [17, 19, 20]. Indeed, “hidden cardiotoxicity” of several drugs may manifest only in the diseased heart, i.e., in the presence of cardiovascular comorbidities and their medications [21]. Therefore optimal risk stratification is essential for reasonable therapy decision in these patients.
An important aspect of risk stratification are cardiovascular biomarkers. Among others, troponin, brain natriuretic peptide (BNP) and NT-terminal proBNP (NT-proBNP) were examined in cancer patients under ICI therapy showing an association with CTR-CVT [17, 22]. As cardiovascular side effects from ICI therapy are mainly mediated by increased inflammatory activity, markers for inflammation may be appropriate for risk stratification [23, 24]. One of those markers is the neutrophil-to-lymphocyte ratio (NLR), easily calculated using absolute cell counts of neutrophils and lymphocytes. This parameter gained recent attention as an inflammatory prognostic marker for mortality and cardiovascular events in patients after COVID-19 infection [25, 26]. Furthermore, it is a predictor for short- and long-term mortality in patients with acute coronary syndromes or heart failure [27–30]. In cancer patients, high NLR values are associated with increased mortality [31]. In the context of CTR-CVT, some studies investigated the association with NLR. Cancer patients with ICI-related CTR-CVT showed higher NLR values, but no specific analysis of its predictive capacity for patients with pre-existing cardiovascular disease was performed [32]. In patients with breast cancer under anthracycline therapy, high NLR values were associated with early signs of CTR-CVT [33]. Increased NLR was associated with major adverse cardiac events (MACE) in patients with ICI myocarditis [34]. There are no studies so far, which examined the prognostic capacity of inflammatory biomarkers for cancer patients with pre-existing cardiovascular diseases. As these patients are at a higher risk to develop CTR-CVT under ICI therapy, the aim of this study was to evaluate a possible prediction of NLR for CTR-CVT in this high-risk population.
Methods
Study population
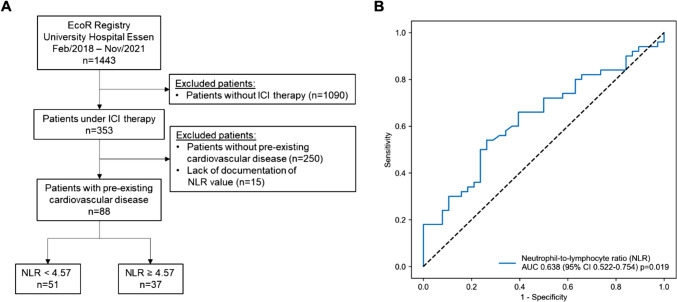
The study population was part of the prospective Essen cardio-oncology registry (ECoR). The registry was approved by the local ethics committee (19-8632-BO) and includes all patients attending to the local outpatient cardio-oncology unit, who provide written informed consent. In this study, cancer patients under ICI therapy with pre-existing cardiovascular disease, like coronary artery disease, congestive heart failure, peripheral or cerebral artery occlusive disease, valvular heart disease, atrial fibrillation or history of stroke were included. Exclusion criteria were no ICI therapy, no pre-existing cardiovascular disease and no documentation of NLR values. From 1443 patients, enrolled between July 2018 and November 2021, we analyzed a subgroup of 88 cancer patient with pre-existing cardiovascular disease treated with ICI therapy. Excluded were 1090 patients not receiving ICI therapy, 250 patients without pre-existing cardiovascular disease and 15 patients due to missing NLR values (Fig. 1A). The local cardio-oncology outpatient unit performed patient enrollment and follow-up. Baseline demographics, cardiovascular history, risk factors, cancer treatment and cancer-related characteristics were recorded. Blood samples were obtained and clinical examination, electrocardiography and echocardiography were performed at patient enrollment. NLR values were calculated as a ratio of absolute neutrophil count to absolute lymphocyte count. According to the optimal predictive NLR cut-off value for CTR-CVT determined by receiver operator characteristic (ROC), we divided the study population into two groups (Fig. 1B). During follow-up, CTR-CVT was assessed using clinical examination, electrocardiography, non-invasive cardiac imaging (echocardiography and cardiac magnetic resonance imaging (CMR)) and measurement of cardiac biomarkers (high-sensitive troponin, brain natriuretic peptide (BNP) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP)). CTRCD, myocarditis, vascular toxicity, new onset of arterial hypertension and arrhythmia or QTc prolongation were defined as CTR-CVT according to the diagnosis criteria of the European guidelines on cardio-oncology [17]. Vascular toxicity was defined as pulmonary embolism, deep venous thrombosis, myocardial infarction and stroke. Arrhythmia was defined as atrial fibrillation and sinus tachycardia besides QTc prolongation. Myocarditis was diagnosed as clinical diagnosis consisting of relevant troponin elevation in combination with positive CMR diagnostic for acute myocarditis and exclusion of CAD progression. The median follow-up was 357 days (interquartile range (IQR): 150–509 days).
Fig. 1.
Flowchart of the study population (A). Out of 1443 patients form the EcoR registry of the University Hospital Essen, 88 cancer patients under immune checkpoint inhibitor (ICI) therapy with pre-existing cardiovascular disease were identified. The study population was further divided into patients with low (< 4.57) and high (≥ 4.57) neutrophil-to-lymphocyte-ratio (NLR) according to the receiver operator characteristic (ROC) curve analysis of NLR for prediction of cancer therapy-related cardiovascular toxicity (B). AUC, area under the curve; CI, confidence interval
Statistical analysis
Categorical variables are given as absolute and relative frequencies (%). Association between these variables was evaluated by the use of chi-squared test. Distribution of continuous variables was assessed using Kolmogorov–Smirnov test. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and Student’s t test was used for comparison. In case of non-normal distribution, continuous variables were displayed as median with IQR. These variables were compared using Mann–Whitney U test. ROC curve analysis was performed for cut-off calculation, and the optimal NLR cut-off value was determined using Youden’s index. The predictive capacity of NLR for overall CTR-CVT was also compared to NT-proBNP and high-sensitive troponin by means of the area under the ROC curve (AUC). Kaplan–Meier cumulative event curves showed overall CTR-CVT in dependence of low and high NLR values and were compared using log-rank test. Patients lost to follow-up were treated as censored observations. Median follow-up time was calculated by the reverse Kaplan–Meier method. Skewed data were logarithmically transformed to improve model stability. The association between logarithmized NLR values (log2(NLR)) and CTR-CVT was examined using an univariable Cox regression. Additionally, different multivariable Cox regression models were computed to show the adjusted association of logarithmized NLR values and overall CTR-CVT. The significance level was set as p < 0.05. All statistical analyses were performed using IBM SPSS 29.0 software (SPSS Inc.). Figures of ROC curve analysis and Kaplan–Meier cumulative event curves were generated with custom Python scripts using appropriate libraries.
Results
In our study population of 88 patients (mean age ± SD = 69 ± 11 years; 25% female; Table 1), melanoma (51.1%) was the most prevalent cancer entity (Table 1). CAD (39.8%) and atrial fibrillation (32.9%) were the most frequent cardiovascular comorbidities (Table 1). NLR values were assessed at time of patient enrollment at the local cardio-oncology outpatient unit. ROC curve analysis with NLR for predicting CTR-CVT resulted in an area under the curve (AUC) of 0.638 (95% confidence interval (CI): 0.522–0.754; p = 0.019; Fig. 1b). This analysis revealed an optimum cut-off value of ≥ 4.57 with 54% sensitivity and 74% specificity. Accordingly, 37 patients (42%) presented with a high NLR value of ≥ 4.57 at time of enrollment. Further, ROC curve analysis illustrated that AUC of NLR is a stronger indicator for overall CTR-CVT than NT-proBNP, but inferior compared to high-sensitive troponin in our study cohort (Supplementary Fig. 1A). Addition of NLR to a model containing NT-proBNP and high-sensitive troponin led to an increase in AUC from 0.619 (NT-proBNP + high-sensitive troponin) to 0.706 (NT-proBNP + high-sensitive troponin + NLR; Supplementary Fig. 1B). Clinical baseline characteristics of the study population according to low and high NLR values are presented in Tables 1 and 2. In the group with low NLR values, more patients suffered from melanoma (31 patients vs. 14 patients; p = 0.034). Despite a significant higher portion of patients with hypertension in the group with low NLR values (44 patients vs. 24 patients; p = 0.018), no differences in co-morbidities between patients with low and high NLR values were observed in this study population. No significant differences in cardiovascular medication were noticeable (Table 2). Concerning cancer manifestation, 61 patients (69.3%) of the study population suffered from metastatic cancer (Table 1). Prior cancer treatment did not differ between patients with low and high NLR values, besides a higher proportion of radiation therapy in patients with high NLR values (10 patients vs. 15 patients; p = 0.032; Table 1). There was no difference in the rate of thoracic radiation therapy. In patients receiving ICI therapy before patient enrollment (61.4%), a new therapy cycle was initiated with a medium of 35 days before patient enrollment. ICI therapy was started in naïve patients with a median of 4 days before patient enrollment. Most of the patients received PD-1 inhibitors as ICI therapy (78 patients, 88.6%) with 48 patients (54.5%) treated with nivolumab and 27 patients (30.7%) with pembrolizumab (Table 2). 17 patients (19.3%) obtained PDL-1 inhibitors with avelumab in the majority (10 patients, 11.4%) and 28 patients (31.8%) received the CTLA-4 inhibitor ipilimumab. More patients with low NLR values were treated with a combination of ipilimumab and a PD-1 inhibitor (20 patients vs. 7 patients; p = 0.042).
Table 1.
Baseline characteristics
| Total (n = 88) | NLR < 4.57 (n = 51) | NLR ≥ 4.57 (n = 37) | p value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 69 ± 11 | 69 ± 12 | 70 ± 11 | 0.582 |
| Sex (female), n (%) | 22 (25.0) | 15 (29.4) | 7 (18.9) | 0.262 |
| BMI, median (IQR) | 26.6 (24.2–30.6) | 27.4 (24.8–30.8) | 25.5 (23.5–29.4) | 0.067 |
| Cancer disease, n (%) | ||||
| Melanoma | 45 (51.1) | 31 (60.8) | 14 (37.8) | 0.034* |
| Merkel cell carcinoma | 10 (11.4) | 5 (9.8) | 5 (13.5) | 0.588 |
| Cutaneous squamous cell carcinoma | 13 (14.8) | 5 (9.8) | 8 (21.6) | 0.123 |
| NSCLC | 12 (13.6) | 6 (11.8) | 6 (16.2) | 0.548 |
| Other | 13 (14.8) | 6 (11.8) | 7 (18.9) | 0.350 |
| Metastasis | 61 (69.3) | 34 (66.7) | 27 (73.0) | 0.828 |
| ≥ 2 cancer entities (in the last 5 years) | 20 (22.7) | 13 (25.5) | 7 (18.9) | 0.468 |
| Cancer progression | 6 (6.8) | 4 (7.8) | 2 (5.4) | 0.601 |
| Cancer recurrence | 22 (25.0) | 16 (31.4) | 6 (16.2) | 0.108 |
| Prior cancer treatment, n (%) | ||||
| Surgery | 73 (83.0) | 42 (82.4) | 31 (83.8) | 0.860 |
| Radiation | 25 (28.4) | 10 (19.6) | 15 (40.5) | 0.032* |
| Thoracic radiation | 9 (10.2) | 5 (9.8) | 4 (10.8) | 0.902 |
| Chemotherapy | 19 (21.6) | 8 (15.7) | 11 (29.7) | 0.114 |
| Anthracycline | 2 (2.3) | 0 | 2 (5.4) | 0.093 |
| Platinum-based chemotherapy | 17 (19.3) | 7 (13.7) | 10 (27.0) | 0.119 |
| Other chemotherapy | 4 (4.5) | 1 (2.0) | 3 (8.1) | 0.172 |
| Tyrosine kinase inhibitor | 5 (5.7) | 2 (3.9) | 3 (8.1) | 0.402 |
| MEK inhibitor | 7 (8.0) | 4 (7.8) | 3 (8.1) | 0.964 |
| B-Raf inhibitor | 6 (6.8) | 4 (7.8) | 2 (5.4) | 0.654 |
| Taxane | 8 (9.1) | 4 (7.8) | 4 (10.8) | 0.633 |
| Prior ICI therapy | 54 (61.4) | 28 (54.9) | 26 (70.3) | 0.144 |
| Cardiovascular comorbidities, n (%) | ||||
| Coronary artery disease | 35 (39.8) | 20 (39.2) | 15 (40.5) | 0.900 |
| Previous PCI | 21 (23.9) | 14 (27.5) | 7 (18.9) | 0.354 |
| CABG | 13 (14.8) | 9 (17.6) | 4 (10.8) | 0.372 |
| Previous myocardial infarction | 12 (13.6) | 7 (13.7) | 5 (13.5) | 0.977 |
| Congestive heart failure | 15 (17.0) | 8 (15.7) | 7 (18.9) | 0.691 |
| Peripheral artery occlusive disease | 9 (10.2) | 4 (7.8) | 5 (13.5) | 0.386 |
| Cerebral artery occlusive disease | 8 (9.1) | 5 (9.8) | 3 (8.1) | 0.785 |
| History of stroke | 20 (22.7) | 14 (27.4) | 6 (16.2) | 0.214 |
| Valvular heart disease | 5 (5.7) | 3 (5.9) | 2 (5.4) | 0.836 |
| Atrial fibrillation | 29 (32.9) | 15 (29.4) | 14 (37.8) | 0.406 |
| Pacemaker | 6 (6.8) | 2 (3.9) | 4 (10.8) | 0.206 |
| Chronic kidney disease | 10 (11.4) | 7 (13.7) | 3 (8.1) | 0.412 |
| COPD | 7 (8.0) | 4 (7.8) | 3 (8.1) | 0.964 |
| Hypertension | 68 (77.3) | 44 (86.3) | 24 (64.9) | 0.018* |
| Diabetes mellitus | 25 (28.4) | 18 (35.3) | 7 (18.9) | 0.093 |
| Dyslipidemia | 28 (31.8) | 19 (37.3) | 9 (24.3) | 0.199 |
| History of smoking (current or past) | 15 (17.0) | 9 (17.6) | 6 (16.2) | 0.917 |
| Obesity | 25 (28.4) | 17 (33.3) | 8 (21.6) | 0.229 |
Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range (IQR)) in case of non-normally distributed data. Categorial variables are shown as frequencies and percentages (%)
NLR neutrophil-to-lymphocyte ratio; BMI body mass index; NSCLC non-small-cell lung cancer; MEK mitogen-activated protein kinase kinase; B-Raf B-rapidly accelerated fibrosarcoma; ICI immune checkpoint inhibitor; PCI percutaneous coronary intervention; CABG coronary artery bypass graft; COPD chronic obstructive pulmonary disease
*Statistically significant difference between cancer patients with low and high NLR
Table 2.
Cardiovascular medication and ICI therapy
| Total (n = 88) | NLR < 4.57 (n = 51) | NLR ≥ 4.57 (n = 37) | p value | |
|---|---|---|---|---|
| Cardiovascular medication, n (%) | ||||
| ASS | 35 (39.8) | 22 (43.1) | 13 (35.1) | 0.463 |
| Statin | 36 (40.9) | 21 (41.2) | 15 (40.5) | 0.975 |
| β blocker | 55 (62.5) | 34 (66.7) | 21 (56.8) | 0.357 |
| ACEi | 32 (36.3) | 18 (35.3) | 14 (37.8) | 0.785 |
| ARB | 21 (23.9) | 15 (29.4) | 6 (16.2) | 0.156 |
| MRA | 10 (11.4) | 5 (9.8) | 5 (13.5) | 0.579 |
| ARNI | 1 (1.1) | 1 (2.0) | 0 | 0.393 |
| Digitalis | 4 (4.5) | 1 (2.0) | 3 (8.1) | 0.169 |
| Oral anticoagulation | 35 (39.8) | 17 (33.3) | 18 (48.6) | 0.321 |
| ICI therapy, n (%) | ||||
| PD-1 | 78 (88.6) | 44 (86.3) | 34 (91.9) | 0.412 |
| Nivolumab | 48 (54.5) | 30 (58.8) | 18 (48.6) | 0.344 |
| Pembrolizumab | 27 (30.7) | 17 (33.3) | 10 (27.0) | 0.527 |
| Cemiplimab | 11 (12.5) | 3 (5.9) | 8 (21.6) | 0.028* |
| Spartalizumab | 1 (1.1) | 1 (2.0) | 0 | 0.392 |
| PDL-1 | 17 (19.3) | 11 (21.6) | 6 (16.2) | 0.530 |
| Avelumab | 10 (11.4) | 6 (11.8) | 4 (10.8) | 0.889 |
| Durvalumab | 2 (2.3) | 2 (3.9) | 0 | 0.223 |
| Atezolizumab | 5 (5.7) | 3 (5.9) | 2 (5.4) | 0.924 |
| CTLA-4 (Ipilimumab) | 28 (31.8) | 20 (39.2) | 8 (21.6) | 0.080 |
| CTLA-4 + PD-1 | 27 (30.7) | 20 (39.2) | 7 (18.9) | 0.042* |
| Duration of ICI therapy (days), median (IQR) | 143 (48–382) | 158 (63–393) | 99 (42–368) | 0.272 |
Data shown as frequencies and percentages (%) or median (interquartile range (IQR)) in case of duration of ICI therapy
NLR neutrophil-to-lymphocyte ratio; ASS acetylsalicylic acid; ACEi angiotensin-converting enzyme inhibitor; ARB angiotensin receptor blocker; MRA mineralocorticoid receptor antagonist; ARNI angiotensin receptor neprilysin inhibitor; PD-1 programmed cell death protein 1; PDL-1 programmed cell death 1 ligand-1; CTLA-4 cytotoxic T lymphocyte-associated antigen-4
*Statistically significant difference between cancer patients with low and high NLR
Baseline laboratory values, assessed at patient enrollment, are depicted in Table 3. The median NLR value was 2.7 in patients with low NLR value and 6.5 in patients with high NLR value. Patients with high NLR value showed higher levels of white blood cell count (6.4/nl vs. 7.9/nl; p = 0.006). High NLR values were driven by a higher count of neutrophils (4.0/nl vs. 5.8/nl; p < 0.001) as well as reduction in lymphocyte count (1.5/nl vs. 0.9/nl; p < 0.001). There were no further significant differences in any laboratory parameters including hemoglobin or inflammatory and cardiac biomarkers between patients with low and high NLR values. Functional cardiac parameters were measured at patient enrollment using electrocardiography (ECG) and echocardiography. Here, no differences were observed between patients with low and high NLR values in this study population (Table 4).
Table 3.
Baseline laboratory parameters
| Total (n = 88) | NLR < 4.57 (n = 51) | NLR ≥ 4.57 (n = 37) | p value | |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 12.6 (10.9–14.1) | 12.6 (11.2–14.1) | 12.5 (10.6–14.2) | 0.663 |
| White blood cell count (count/nl) | 7.0 (5.6–9.3) | 6.4 (5.3–8.3) | 7.9 (6.2–11.3) | 0.006* |
| Neutrophils (count/nl) | 4.6 (3.7–6.8) | 4.0 (3.3–5.1) | 5.8 (4.6–8.6) | < 0.001* |
| Lymphocytes (count/nl) | 1.3 (0.9–1.7) | 1.5 (1.2–1.9) | 0.9 (0.7–1.3) | < 0.001* |
| NLR | 4.2 (2.5–6.2) | 2.7 (2.0–3.9) | 6.5 (5.4–7.8) | < 0.001* |
| CRP (mg/dl) | 0.7 (0.4–2.3) | 0.5 (0.4–1.7) | 0.9 (0.4–2.9) | 0.224 |
| Monocytes (count/nl) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.6 (0.5–0.9) | 0.291 |
| Platelets (count/nl) | 225.5 (191.3–286.0) | 221.0 (191.0–264.0) | 232.0 (186.0–316.0) | 0.548 |
Parameters are displayed as median (interquartile range (IQR))
NLR neutrophil-to-lymphocyte ratio; CRP C-reactive protein
*Statistically significant difference between cancer patients with low and high NLR
Table 4.
Functional cardiac parameters at baseline
| Total (n = 88) | NLR < 4.57 (n = 51) | NLR ≥ 4.57 (n = 37) | p value | |
|---|---|---|---|---|
| ECG parameters | ||||
| Heart rate (bpm) | 78 ± 16 | 76 ± 17 | 81 ± 15 | 0.191 |
| QRS (ms) | 92.0 (83.5–106.5) | 94.0 (82.0–106.0) | 92.0 (86.0–114.0) | 0.530 |
| QTc (ms) | 443.6 ± 34.0 | 443.0 ± 35.8 | 444.5 ± 31.7 | 0.851 |
| Echocardiography | ||||
| LV-EF (%) | 55.2 ± 8.6 | 56.8 ± 7.2 | 53.1 ± 10.0 | 0.059 |
| LV GLS (%) | − 19.7 (− 22.1 to − 16.7) | − 19.8 (− 22.3 to − 17.1) | − 19.6 (− 20.8 to − 13.7) | 0.203 |
| LAVI (ml/m2) | 29.3 ± 12.7 | 29.5 ± 12.7 | 29.1 ± 12.8 | 0.890 |
| E/E' (ratio) | 10.5 ± 3.4 | 10.2 ± 3.1 | 10.8 ± 4.0 | 0.707 |
| sPAP (mmHg) | 32.9 ± 10.8 | 30.8 ± 8.5 | 35.9 ± 13.1 | 0.098 |
| TAPSE (mm) | 24.3 ± 5.9 | 24.2 ± 6.0 | 24.3 ± 5.9 | 0.975 |
Variables are expressed as mean ± standard deviation (SD) or median (interquartile range (IQR)) in case of non-normally distributed data
NLR neutrophil-to-lymphocyte ratio; LV-EF left ventricular ejection fraction; LV GLS left ventricular global longitudinal strain; LAVI left atrium volume index; sPAP systolic pulmonary artery pressure; TAPSE tricuspid annular plane systolic excursion
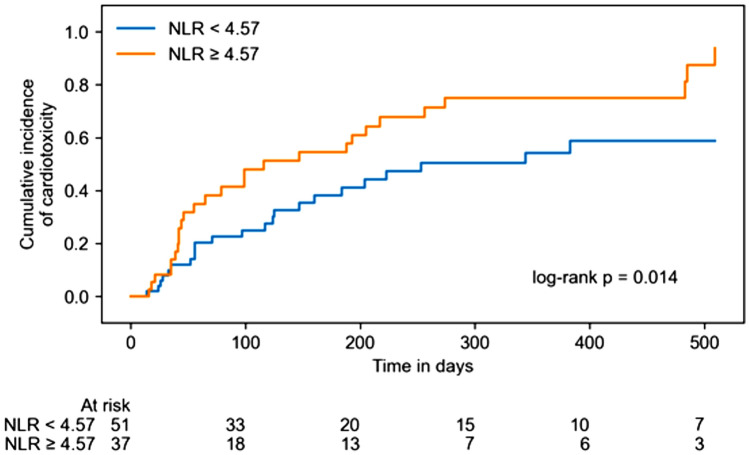
Overall CTR-CVT, consisting of CTRCD, myocarditis, vascular toxicity, new onset of arterial hypertension and arrhythmia or QTc prolongation, occurred in 50 of the 88 patients (56.8%) (17 patients with CTRCD (19.3%), 4 patients with myocarditis (4.5%), 17 patients with vascular toxicity (19.3%), 3 patients with new onset of arterial hypertension (3.4%) and 22 patients with arrhythmia or QTc prolongation (25.0%); Table 5). 14 patients (15.9%) showed more than one cardiotoxic entity. Univariable Cox regression analysis of logarithmized NLR values at patient enrollment showed a moderate association to increased overall CTR-CVT (hazard ratio (HR): 1.443; 95% CI: 1.082–1.925; p = 0.013). This was mainly attributable to a significant association of NLR with myocarditis (HR: 5.556; 95% CI: 2.077–14.861); p < 0.001) and arrhythmia or QTc prolongation (HR: 1.741; 95% CI: 1.161–2.610; p = 0.007). NLR values ≥ 4.57 were significantly associated with overall CTR-CVT (log-rank p = 0.014; Fig. 2). The association of logarithmized NLR values with arrhythmia events was mainly referable to a significant association with atrial fibrillation (HR: 2.107; 95% CI: 1.311–3.389; p = 0.002; Supplementary Table 1) and QTc prolongation (HR: 2.260; 95% CI: 1.064–4.802; p = 0.034; Supplementary Table 1). In ICI therapy naïve patients, univariate Cox regression analysis showed a significant association of logarithmized NLR values with overall CTR-CVT (HR: 2.563; 95% CI 1.074–6.116; p = 0.034; Supplementary Table 2).
Table 5.
Univariable Cox regression for log2(NLR)
| Outcome | Number of events | Estimated hazard ratio (95% CI) | p value |
|---|---|---|---|
| Overall CTR-CVT | 50 | 1.443 (1.082–1.925) | 0.013* |
| CTRCD | 17 | 1.448 (0.799–2.626) | 0.222 |
| Myocarditis | 4 | 5.556 (2.077–14.861) | < 0.001* |
| Vascular toxicity | 17 | 1.314 (0.794–2.175) | 0.288 |
| New onset of arterial hypertension | 3 | 1.994 (0.682–5.826) | 0.207 |
| Arrhythmia or QTc prolongation | 22 | 1.741 (1.161–2.610) | 0.007* |
NLR neutrophil-to-lymphocyte ratio; CI confidence interval; CTR-CVT cancer therapy-related cardiovascular toxicity; CTRCD cancer therapy-related cardiovascular dysfunction
*Statistically significant association between NLR and outcome
Fig. 2.
Kaplan–Meier cumulative event curves for overall cancer therapy-related cardiovascular toxicity (CTR-CVT) with patients separated by low (< 4.57) and high (≥ 4.57) neutrophil-to-lymphocyte ratios (NLR)
Several multivariable Cox regression analyses were performed to adjust for different baseline characteristics. The association of NLR with overall CTR-CVT remained significant after adjustment for age and sex (HR: 1.441; 95% CI: 1.069–1.942; p = 0.016; Table 6 – multivariable model 1). Similar results were observed after further adjustment for cardiovascular risk factors and comorbidities, including hypertension, dyslipidemia, diabetes mellitus, obesity, history of smoking, CAD, congestive heart failure, valvular heart disease, coronary artery bypass graft (CABG), peripheral and cerebral artery occlusive disease, previous myocardial infarction, percutaneous coronary intervention (PCI) or stroke and atrial fibrillation (HR: 1.464; 95% CI: 1.028–2.084; p = 0.034; Table 6, multivariable model 2). However, this significant association was lost after additional adjustment for cancer entities of the examined patients (HR: 1.299; 95% CI: 0.814–2.073; p = 0.272; Table 6, multivariable model 3).
Table 6.
Multivariable Cox Regression for log2(NLR)
| Outcome | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Estimated hazard ratio (95% CI) | p value | Estimated hazard ratio (95% CI) | p value | Estimated hazard ratio (95% CI) | p value | |
| Overall CTR-CVT | 1.441 (1.069–1.942) | 0.016* | 1.464 (1.028–2.084) | 0.034* | 1.299 (0.814–2.073) | 0.272 |
Model 1: Adjustment for age and sex; Model 2: Adjustment for age, sex, hypertension, dyslipidemia, diabetes mellitus, obesity, history of smoking, coronary artery disease (CAD), congestive heart failure, valvular heart disease, coronary artery bypass graft (CABG), peripheral and cerebral artery occlusive disease, previous myocardial infarction, percutaneous coronary intervention (PCI) or stroke and atrial fibrillation; Model 3: Adjustment for age, sex, hypertension, dyslipidemia, diabetes mellitus, obesity, history of smoking, coronary artery disease (CAD), congestive heart failure, valvular heart disease, coronary artery bypass graft (CABG), peripheral and cerebral artery occlusive disease, previous myocardial infarction, percutaneous coronary intervention (PCI) or stroke, atrial fibrillation and cancer entities
NLR neutrophil-to-lymphocyte ratio; CI confidence interval; CTR-CVT cancer therapy-related cardiovascular toxicity
*Statistically significant association between NLR and outcome
Discussion
This study examined the association of NLR with CTR-CVT in cancer patients under ICI therapy with pre-existing cardiovascular disease. NLR was significant associated with overall CTR-CVT, mainly driven by the incidence of myocarditis and arrhythmia or QTc prolongation. This association remains after adjustment for age, sex, cardiovascular risk factors and co-morbidities. However, the significance was lost after further adjustment for cancer entities.
Compared to the incidence of CTR-CVT under ICI therapy in general, a relatively high proportion of patients (56.8%) included in our study developed a cardiotoxic event. Several studies mostly reported a CTR-CVT incidence of 6–7% under ICI therapy [35, 36]. These studies mainly defined CTR-CVT as cardiac immune-related adverse events (irAE), including myocarditis, acute coronary syndrome, Takotsubo syndrome and pericardial disease. We investigated the whole spectrum of CTR-CVT, recently defined by the European guidelines on cardio-oncology [17]. Therefore, we additionally included some rather mild diagnosis criteria for CTR-CVT, like cardiac dysfunction, new onset of arterial hypertension or arrhythmia. This might result in a higher incidence of CTR-CVT in our collective. Furthermore, the patients investigated in this study are severe diseased and at higher risk developing CTR-CVT and therefore more likely to attend to our local cardio-oncology outpatient unit, which creates a selection bias. This might explain a higher incidence of myocarditis (4.5%), observed in our study, as other studies reported an incidence of ICI-related myocarditis up to 1% [37, 38]. So far, there are no specific trials estimating the incidence of cardiotoxic events in these high-risk cancer patients with pre-existing cardiovascular disease. Our study population is comparably small; therefore, larger trials are needed to portray a more representative incidence of CTR-CVT in these patients.
Although, sensitivity and specificity of NLR for prediction of CTR-CVT in our study cohort were not ideal, they increased the diagnostic validity of the established parameters like NT-proBNP and high-sensitive troponin, highlighting the additional benefit of this economically biomarker. Also, an AUC of 0.63 appears moderate for the prediction of overall CTR-CVT, but compared to other studies, investigating the association of NLR with cardiotoxicity under anti-cancer treatment similar values for AUC is reported [33]. The same applies to studies examining the association of NLR with mortality or myocardial injury in other non-cancer collectives [39–41].
These results further confirm the association of NLR with CTR-CVT as reported before in other studies. In patients with breast cancer under anthracycline therapy, NLR was associated with deterioration of left ventricular global longitudinal strain (GLS) [33]. High NLR values were also observed in patients with ICI-related myocarditis and arrhythmia [32, 42]. Monitoring of NLR in patients under anti-PD1 therapy was predictive for immune-related adverse events in general [43]. In patients with ICI therapy-related myocarditis, high NLR was also associated with major adverse cardiac events (MACE) [34]. Association of NLR with CTR-CVT was also described in lung cancer patients after initiation of ICI therapy [44]. However, one study showed unaltered NLR in patients developing CTR-CVT under ICI therapy [35]. These patients primarily suffered from respiratory or gastrointestinal tumor disease. The only CTR-CVT endpoint in this study was myocarditis, whereas our study included all entities of CTR-CVT described by the European guidelines on cardio-oncology. In addition, our study population differed considerably, as the majority suffered from melanoma and other skin cancer entities. Apart from cancer patients, there are studies demonstrating a predictive capacity of NLR with cardiovascular injury in a variety of diseases. There is an association of NLR with severity of myocarditis reported [45]. In patients with acute coronary syndrome, NLR showed predictive capacity for myocardial damage and cardiac dysfunction as well as arrhythmia [46, 47]. In other studies, high NLR forecasted the onset of atrial fibrillation [48–50]. Moreover, high NLR values were observed in patients with QT prolongation [51].
High NLR values certainly reflect high inflammatory activity [52]. This is not exclusively observed in patients under ICI therapy and therefore not specific for prediction of CTR-CVT in ICI recipients. Previous studies, however, have concluded that NLR may predict cardiotoxicity in breast cancer patients under anthracycline treatment [33]. In addition, NLR is associated with mortality in cancer patients independent of anti-cancer treatment [53–55]. Furthermore, NLR is a surrogate marker of poor outcomes in the field of cardiovascular disease [27–29]. In our study, NLR is associated with overall CTR-CVT in high-risk cancer patients under ICI therapy highlighting high inflammatory activity as a possible mediator for CTR-CVT in these patients.
Several possible underlying mechanisms are reported for pro-arrhythmic properties of neutrophils in other cardiac disease models. After myocardial infarction, lipocalin-2 (Lcn2)-mediated production of reactive oxygen species (ROS) promotes ventricular arrhythmia [56]. Production of neutrophil extracellular traps (NETs) contributes to progression of atrial fibrillation [57]. Furthermore, the integrin CD11b might be linked to atrial fibrosis and the induction of atrial fibrillation [58]. These mechanisms could be responsible for the association of NLR with arrhythmia and should be subject of further investigations.
High NLR values observed in our study population seemed to be driven by an increase of neutrophils and decrease of lymphocytes. Higher levels of neutrophils reflect a systemic inflammatory status leading to the production of reactive oxygen species and pro-inflammatory cytokines, which can promote myocardial damage [59–62]. In addition, elevated levels of cortisol during a stressed state are associated with a reduction in lymphocyte count, which is accompanied by enhanced tissue injury [63]. These two conditions are able to cause myocardial damage reflecting in an elevated NLR associated with CTR-CVT in our study population.
This study has several limitations. Cardiotoxic confounders like time interval between previous cancer therapies, whether radiation or chemotherapy, were not examined. The same applies for non-cardiovascular premedication apart from reported cancer treatment. Furthermore, our study does not provide any information regarding therapy with corticosteroids as an influencing factor of neutrophil and lymphocyte count in the blood [64, 65]. However, the main portion of included patients suffered from melanoma. For these patients, steroids are mostly used for management of irAE [66]. In our study, only 6.8% of included patients presented with irAE at patient enrollment and could therefore be possibly treated with steroids before study inculsion (Supplementary Table 3). We also analyzed a relatively small number of heterogeneous patients. Therefore, larger prospective clinical trials are needed.
Conclusion
NLR was associated with overall cancer therapy-related cardiovascular toxicity in cancer patients with pre-existing cardiovascular disease under ICI therapy, representing a high-risk patient collective. The association was mainly driven by the incidence of myocarditis and arrhythmia or QTc prolongation. However, after adjustment for further confounders, this association lost its significance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the use of elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by German Cardiac Society (DGK, Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e.V.) under Grant DGK02/2022 and Universitätsmedizin Essen Clinical Scientist Acedemy (UMEA) Junior Fellowship to E.H.-Y.. The German Research Foundation also supported this work under Grant RA969/12–1 to T.R.. P.F. was supported by the National Research, Development and Innovation Office of Hungary (Research Excellence Program TKP within the framework of the Therapeutic Development thematic program of the Semmelweis University; National Heart Laboratory (RRF-2.3.1–21-2022–00003). P.F. is a vice chair of the COST CIG (IG16225) focusing on cardioprotection.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
M.T. and T.R. report personal fees and others from Edwards and Novartis, Bristol Myers Squibb, Bayer, Daiichi Sankyo and Astra Zeneca, which are outside the submitted work. T.R. cofounded Bimyo, a company focusing on the development of cardioprotective peptides. All other authors declare no conflict of interest. P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies holding patents on cardioprotective oligonucleotides and providing R&D services for drug development.
Ethical standards
All procedures were in accordance with the ethical standards of the institutional ethics committees of the participating centers and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.El Helali A, et al. A meta-analysis with systematic review: efficacy and safety of immune checkpoint inhibitors in patients with advanced gastric cancer. Front Oncol. 2022;12:908026. doi: 10.3389/fonc.2022.908026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu X, et al. Immune checkpoint inhibitors and survival outcomes in brain metastasis: a time series-based meta-analysis. Front Oncol. 2020;10:564382. doi: 10.3389/fonc.2020.564382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamba N, Ott PA, Iorgulescu JB. Use of first-line immune checkpoint inhibitors and association with overall survival among patients with metastatic melanoma in the anti-PD-1 era. JAMA Netw Open. 2022;5(8):e2225459. doi: 10.1001/jamanetworkopen.2022.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn B, et al. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer. 2020;126(5):978–985. doi: 10.1002/cncr.32624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu A, et al. Immune checkpoint inhibitors and long-term survival of patients with metastatic urothelial cancer. JAMA Netw Open. 2023;6(4):e237444. doi: 10.1001/jamanetworkopen.2023.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michel L, Rassaf T, Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasc. 2019;25:100420. doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferris RL, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nghiem P, et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer. 2021;9(4):e002478. doi: 10.1136/jitc-2021-002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Schilsky RL, et al. Progress in cancer research, prevention, and care. N Engl J Med. 2020;383(10):897–900. doi: 10.1056/NEJMp2007839. [DOI] [PubMed] [Google Scholar]
- 13.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 14.Gan L, et al. Cardiotoxicity associated with immune checkpoint inhibitors: current status and future challenges. Front Pharmacol. 2022;13:962596. doi: 10.3389/fphar.2022.962596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Bhatti SA, Ying J. Immune checkpoint inhibitors-associated cardiotoxicity. Cancers (Basel) 2022;14(5):1145. doi: 10.3390/cancers14051145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel L, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. 2022;43(4):316–329. doi: 10.1093/eurheartj/ehab430. [DOI] [PubMed] [Google Scholar]
- 17.Lyon AR, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann J, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battisti NML, et al. Prevalence of cardiovascular disease in patients with potentially curable malignancies: a national registry dataset analysis. JACC CardioOncol. 2022;4(2):238–253. doi: 10.1016/j.jaccao.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousif LI, et al. Risk factors for immune checkpoint inhibitor-mediated cardiovascular toxicities. Curr Oncol Rep. 2023;25(7):753–763. doi: 10.1007/s11912-023-01414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferdinandy P, et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J. 2019;40(22):1771–1777. doi: 10.1093/eurheartj/ehy365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. 2018;10(Suppl 35):S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252(4):283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 24.Ravindranathan D, Master VA, Bilen MA. Inflammatory markers in cancer immunotherapy. Biology (Basel) 2021;10(4):325. doi: 10.3390/biology10040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan L, et al. Predictive value of neutrophil/lymphocyte ratio (NLR) on cardiovascular events in patients with COVID-19. Int J Gen Med. 2021;14:3899–3907. doi: 10.2147/IJGM.S317380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higaki A, et al. Predictive value of neutrophil-to-lymphocyte ratio for the fatality of COVID-19 patients complicated with cardiovascular diseases and/or risk factors. Sci Rep. 2022;12(1):13606. doi: 10.1038/s41598-022-17567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat T, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 28.Qiao S, Gao W, Guo S. Neutrophil-lymphocyte ratio (NLR) for predicting clinical outcomes in patients with coronary artery disease and type 2 diabetes mellitus: a propensity score matching analysis. Ther Clin Risk Manag. 2020;16:437–443. doi: 10.2147/TCRM.S244623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaya MG, et al. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol. 2013;168(2):1154–1159. doi: 10.1016/j.ijcard.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 30.Cho JH, et al. Neutrophil-lymphocyte ratio in patients with acute heart failure predicts in-hospital and long-term mortality. J Clin Med. 2020;9(2):557. doi: 10.3390/jcm9020557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinci Ozyurek B, et al. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18(5):1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang L, et al. Inflammatory biomarkers in assessing severity and prognosis of immune checkpoint inhibitor-associated cardiotoxicity. ESC Heart Fail. 2023;10(3):1907–1918. doi: 10.1002/ehf2.14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baruch R, et al. High neutrophil-to-lymphocyte ratio as an early sign of cardiotoxicity in breast cancer patients treated with anthracycline. Clin Cardiol. 2023;46(3):328–335. doi: 10.1002/clc.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drobni ZD, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc. 2020;9(23):e018306. doi: 10.1161/JAHA.120.018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, et al. Immune checkpoint inhibitor-associated cardiotoxicity in solid tumors: real-world incidence, risk factors, and prognostic analysis. Front Cardiovasc Med. 2022;9:882167. doi: 10.3389/fcvm.2022.882167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Souza M, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42(16):1621–1631. doi: 10.1093/eurheartj/ehaa884. [DOI] [PubMed] [Google Scholar]
- 37.Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmood SS, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He S, et al. Correlation between neutrophil to lymphocyte ratio and myocardial injury in population exposed to high altitude. Front Cardiovasc Med. 2021;8:738817. doi: 10.3389/fcvm.2021.738817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurdal A, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with myocardial infarction with non-obstructive coronary arteries. Angiology. 2020;71(9):812–816. doi: 10.1177/0003319720938621. [DOI] [PubMed] [Google Scholar]
- 41.Ji Z, et al. The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovasc Med. 2021;8:706852. doi: 10.3389/fcvm.2021.706852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie X, et al. Multi-organ immune-related adverse event is a risk factor of immune checkpoint inhibitor-associated myocarditis in cancer patients: a multi-center study. Front Immunol. 2022;13:879900. doi: 10.3389/fimmu.2022.879900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsukane R, et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep. 2021;11(1):1324. doi: 10.1038/s41598-020-79397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moey MYY, et al. Characterization of immune checkpoint inhibitor-related cardiotoxicity in lung cancer patients from a rural setting. JACC CardioOncol. 2020;2(3):491–502. doi: 10.1016/j.jaccao.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirna M, et al. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. 2021;11(1):18101. doi: 10.1038/s41598-021-97678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7(2):192–199. doi: 10.1016/j.imr.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14(5):573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 48.Gibson PH, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105(2):186–191. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Shao Q, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. 2015;46(3):199–206. doi: 10.1016/j.arcmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Guo X, et al. Postablation neutrophil/lymphocyte ratio correlates with arrhythmia recurrence after catheter ablation of lone atrial fibrillation. Chin Med J (Engl) 2014;127(6):1033–1038. [PubMed] [Google Scholar]
- 51.Ozyilmaz S, et al. The importance of the neutrophil-to-lymphocyte ratio in patients with hypertrophic cardiomyopathy. Rev Port Cardiol. 2017;36(4):239–246. doi: 10.1016/j.repc.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Kourilovitch M, Galarza-Maldonado C. Could a simple biomarker as neutrophil-to-lymphocyte ratio reflect complex processes orchestrated by neutrophils? J Transl Autoimmun. 2023;6:100159. doi: 10.1016/j.jtauto.2022.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ethier JL, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang J, et al. Neutrophil-to-lymphocyte ratio and risk of lung cancer mortality in a low-risk population: a cohort study. Int J Cancer. 2019;145(12):3267–3275. doi: 10.1002/ijc.32640. [DOI] [PubMed] [Google Scholar]
- 55.Iwai N, et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. 2020;10(1):18758. doi: 10.1038/s41598-020-75745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grune J, et al. Neutrophils incite and macrophages avert electrical storm after myocardial infarction. Nat Cardiovasc Res. 2022;1(7):649–664. doi: 10.1038/s44161-022-00094-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He L, et al. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct Target Ther. 2023;8(1):279. doi: 10.1038/s41392-023-01497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedrichs K, et al. Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PLoS ONE. 2014;9(2):e89307. doi: 10.1371/journal.pone.0089307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carbone F, Bonaventura A, Montecucco F. Neutrophil-related oxidants drive heart and brain remodeling after ischemia/reperfusion injury. Front Physiol. 2019;10:1587. doi: 10.3389/fphys.2019.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Kazzi M, et al. Neutrophil-mediated cardiac damage after acute myocardial infarction: significance of defining a new target cell type for developing cardioprotective drugs. Antioxid Redox Signal. 2020;33(10):689–712. doi: 10.1089/ars.2019.7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veenith T, et al. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci Rep. 2022;12(1):10484. doi: 10.1038/s41598-022-13825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biswas M, et al. The mechanistic role of neutrophil lymphocyte ratio perturbations in the leading non communicable lifestyle diseases. F1000Res. 2022;11:960. doi: 10.12688/f1000research.123245.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronchetti S, et al. How Glucocorticoids Affect the Neutrophil Life. Int J Mol Sci. 2018;19(12):4090. doi: 10.3390/ijms19124090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sackstein R, Borenstein M. The effects of corticosteroids on lymphocyte recirculation in humans: analysis of the mechanism of impaired lymphocyte migration to lymph node following methylprednisolone administration. J Investig Med. 1995;43(1):68–77. [PubMed] [Google Scholar]
- 66.Bar-Hai N, et al. Better late than never: the impact of steroidal treatment on the outcome of melanoma patients treated with immunotherapy. Cancers (Basel) 2023;15(11):3041. doi: 10.3390/cancers15113041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study is available from the corresponding author upon reasonable request.