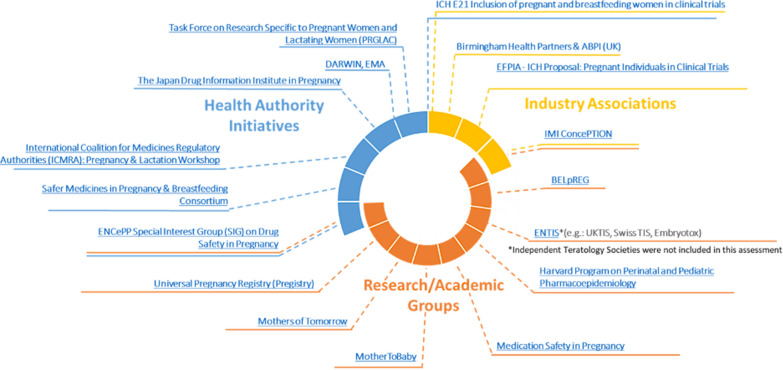
Figure 2.
Pregnancy and breastfeeding initiatives from health authorities, industry associations, and research/academic groups. Relevant initiatives include the Association of the British Pharmaceutical Industry (ABPI) Maternal Health Project Group [8], the BELgian interdisciplinary initiative to enhance pregnancy related data REGistration and research on medication use (BELpREG) [43], the European Federation of Pharmaceutical Industries and Associations (EFPIA)—International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Proposal on Pregnant Individuals in Clinical Trials [44], the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Special Interest Group on Drug Safety and Pregnancy [45], the European Network of Teratology Information Services (ENTIS) [46], the Innovative Medicines Initiative (IMI) ConcePTION [7], the United States Task Force on Research Specific to Pregnant Women and Lactating Women [9], the Japan Drug Information Institute in Pregnancy [47], the International Coalition for Medicines Regulatory Authorities (ICMRA) Pregnancy and Lactation Workshop [48], the United Kingdom (UK) Safer Medicines in Pregnancy and Breastfeeding Consortium [49], the Universal Pregnancy Registry (Pregistry) [50], Mothers of Tomorrow [51], MotherToBaby [52], Medication Safety in Pregnancy [53], and the Harvard Program on Perinatal and Pediatric Pharmacoepidemiology [54].