Abstract
Objective
To study the role and mechanism of chloride channel-3 (ClC-3) in the formation of hypertrophic scar by constructing ClC-3 interference vectors and examining their effects on human hypertrophic scar fibroblasts (HSFB).
Methods
Human HSFB and human normal skin fibroblasts (NSFB) were used in this study, and ClC-3 interference vectors were constructed to transfect cells. ClC-3 inhibitors NPPB and Tamoxifen were used to treat cells. Cell migration and the expression of TGF-β/Smad, CollagenⅠ,CollagenⅢ were examined to explore the role of ClC-3 in the formation of hypertrophic scar.
Results
Compared with the normal skin tissue, the positive expression of ClC-3 and TGF–β in the scar tissue was significantly increased. The relative expression of ClC-3 and TGF-β1 in HSFB cells was higher than that in NSFB cells. Interfering with the expression of CLC-3 can inhibit the migration of HSFB cells and the expression of TGF- β/Smad, CollagenⅠ/Ⅲ. The experiment of HSFB cells treated by CLC-3 inhibitors can also obtain similar results.
Conclusion
Inhibiting CLC-3 can reduce the formation of hypertrophic scars.
Keywords: Hypertrophic scar, HSFB, ClC-3, TGF-β1/Smad3
1. Introduction
Hypertrophic scar shows excessive dermal fibrosis, which is caused by inflammatory cell infiltration, excessive cell proliferation and collagen synthesis during wound healing [1]. Skin tension and wound infection are the main causes of scar formation. Local inflammatory reaction is also an important factor affecting scar hyperplasia. Reduction in inflammation helps to reduce scar hyperplasia [2]. The hypertrophic scar caused by trauma and surgery is a problem for most patients, so it is of great significance to explore the mechanisms underlying hypertrophic scar.
Transforming growth factor- β (TGF- β) It is a major factor that triggers the process of fibrosis [3,4]. TGF-β induces the expression of fibrosis related gene such as α-SMA and collagen through both classical and non-classical signaling pathways [3,5]. Transforming growth factor-β1 (TGF-β1) can promote fibroblast migration and proliferation, and increase collagen synthesis and deposition. Scar formation is the result of skin fibrosis driven by myofibroblasts. Therefore, the formation of hypertrophic scar is considered to be one of the main causes of TGF-β1 activation and secretion [6]. TGF-β/Smad signaling pathway is a key signaling pathway that regulates collagen formation in fibroblasts and myofibroblasts. Sustained activation of TGF-β/Smad signaling pathway leads to long-term overactivation of fibroblasts and myofibroblasts and promotes the production of extracellular matrix (ECM). This is the main reason for excessive collagen formation in hyperplastic scars [6]. In view of the regulatory role of TGF-β/Smad signaling pathway in fibroblasts and myofibroblasts, there are two major therapeutic strategies to reduce hyperplastic scars: make the cell disfunction and inhibit cell proliferation and migration [6,7].
Chloride channel 3 (ClC-3) is a member of the chloride channel superfamily and a multifunctional protein that plays a key role in regulating ion homeostasis, vesicle acidification and membrane excitability [8]. peng et al. found that there was a close relationship between ClC-3 chloride channel and TGF-β1. The interference of ClC-3 could inhibit the activation of TGF-β/Smad signaling pathway [9]. Therefore, ClC-3 may be a potential target for the treatment of hyperplastic scars. we hypothesized that ClC-3 may be one of the potential factors regulating hypertrophic scar formation. In order to explore the role of CLC-3 in the process of hypertrophic scar formation, HSFB cell migration and collagen expression were observed after interference with the expression of CLC-3.
2. Materials and methods
2.1. Cells and tissues
Human hypertrophic scar fibroblasts (HSFB), human normal skin fibroblasts (NSFB) (HUM-iCell-s035, iCell Bioscience Inc, Shanghai); Hypertrophic scar tissue samples, normal dermal tissue samples (The Second Affiliated Hospital of Guangxi Medical University).
2.2. Reagents
Tamoxifen (HY-13757A, MCE); NPPB (HY-101012, MCE); ClC-3 interference lentivirus vector, ClC-3 interference lentivirus empty vector (Zhonghong Boyuan Molecular Laboratory); anti-ClC-3 (bs-6981R, Bioss, 1/200); anti-TGF-β1 (AF1027, Affinity, 1/100); anti-Smad3 (AF6362, Affinity, 1/100).
2.3. Cell culture and transfection
Cells were resuscitated and cultured in the CO2 incubator (BPN–80CW, Shanghai Yiheng Scientific Instrument Co., Ltd.). The cultured medium was changed according to the cell state the next day. Cells were passaged at the ratio of 1:3 when the cell confluence reached 80–90 %. The cells were plated on 6-well plates (2–10 × 105 cells per well) or 6-cm dishes (5 × 105–2 × 106 cells) and cultured in the incubator till the cells completely adhered to the wall. Take 6-well plate as an example (the volume of culture medium in 6-cm dish is twice that of 6-well plate), cells were subjected to transfection when the cell confluence reached 70 %. Before transfection, the cell culture medium was replaced with 1 ml of serum-free culture medium. Two sterilized EP tubes were prepared, and 125 μl Opti-MEM was added to each tube. 5 μl of lipofectamine 3000 was added to one tube, and 12.5 μl of siRNA was added to the other EP tube (siRNA powder was dissolved in DEPC water; 125 μl/1OD). After incubation at room temperature for 5 min, the solution in each EP tube was mixed and incubated at room temperature for 15 min. The mixed solution was added into the 6-well plate and cells were cultured in the incubator. 4–6 h following transfection, 1 ml of complete medium containing 20 % serum was added to the 6-well plate, and experiments were carried out 48 h later.
2.4. Lentivirus infection
Take the 6-well plate as an example, the medium was replaced and 1 ml of complete medium was added when cell confluence was about 30–50 %. According to the pre-experiment, virus was added to incubate with cells at an MOI of 100. An appropriate amount of polybrene (5 μg/ml) was then added and replenished with the complete medium (1 ml) after 4–6 h of infection. The second day after cell infection, the culture medium containing virus was discarded and replaced with fresh complete culture medium. After 48–72 h of virus infection, the follow-up experiments were conducted.
2.5. Scratch test
Scratch was made by 10 μl/200 μl pipette tip when the cell confluence reached more than 90 %. The culture medium was discarded, and cells were washed with PBS for three times. Serum-free culture medium was then added, and pictures of scratches were taken. The cells were incubated in the incubator, and pictures of the scratches were taken again 24 h later. The migration rate of the cells was calculated.
2.6. Immunofluorescence
The paraffin sections were dewaxed, and the sealing liquid was sucked out. The diluted primary antibody (1:200) was dropped into the culture dish, and incubated at 4 °C overnight. After washing with PBS for 3 times, 3 min each time, the excess liquid in the culture dish was sucked out. The diluted fluorescent secondary antibody (1:200) was then added and incubated at 37 °C for 30 min. Cells were washed with PBS for 3 times, 3 min each time. DAPI was dripped and incubated in the dark for 5 min to stain the nuclei. The excess DAPI was washed with PBS. The culture dish was sealed with 50 % glycerin, and then observed under the inverted fluorescence microscope (MF53, Guangzhou Mingmei Photoelectric Co., Ltd.).
2.7. Immunohistochemistry
The tissue sections were baked, dewaxed and hydrated. Antigen was repaired with citric acid buffer. After blocking with 5 % BSA, the sections were incubated with primary antibodies (1:100) at 4 °C overnight, followed by incubation with the second antibody (1:100) labeled with horseradish peroxidase. Subsequently, DAB color development, hematoxylin counterstaining, dehydration and transparent were conducted. The sections were then sealed and observed under the microscope (BX43, Olympus).
2.8. Western blot
The cells were collected, total protein was extracted with RIPA lysate and then centrifuged at 12,000 r/min at 4 °C for 10 min. The supernatant was taken and the total protein was quantified with a BCA protein quantitative kit. After denaturation, the protein samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and membrane transfer with constant flow of 300 mA. The PVDF membrane (Millipore) was blocked with skimmed milk, incubated with the primary antibody at 4 °C overnight, followed by incubation with the secondary antibody at room temperature. The PVDF membrane was soaked with luminescent liquid, and then placed in the ultra-high sensitivity chemiluminescence imaging system (Chemi DocTM XRS+, Bio-Rad Laboratories (Shanghai) Co., Ltd.) for development.
2.9. qPCR detection
Trizon reagent was used to extract total RNA from cells. RNA ultrapure extraction kit and miRNA ultrapure extraction kit were used to extract mRNA and miRNA. The concentration and purity of mRNA were determined by UV–visible spectrophotometer. cDNA was synthesized by RNA reverse transcription kit and miRNA reverse transcription kit. Primers used in this study were shown in Table 1. The quantitative PCR was performed by the fluorescence PCR instrument, and the reaction steps were as follows: pre-denaturation at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 30 s and extension at 72 °C for 30 s.
Table 1.
Primers used in this study.
| Primer name | Sequences (5′-3′) |
|---|---|
| β-actin F | AGGGAAATCGTGCGTGAC |
| β-actin R | CATACCCAAGAAGGAAGGCT |
| ClC-3 F | CATTTGCCTTAGTGCGTTGT |
| ClC-3 R | ATGATATAAGAACCAGGACCCTCT |
| TGF-β1 F | CCGACTACTACGCCAAGGA |
| TGF-β1 R | AACCACTGCCGCACAACTC |
| Smad3 F | GAGGAGAAATGGTGCGAGAA |
| Smad3 R | CAGGCGGCAGTAGATGACA |
| Collagen Ⅰ F | AGACATCCCACCAATCACCT |
| Collagen Ⅰ R | CGTCATCGCACAACACCTT |
| Collagen Ⅲ F | TGGCATCAAAGGACATCG |
| Collagen Ⅲ R | CATAATACGGGGCAAAACC |
2.10. Statistical analysis
All data were statistically analyzed by IBM SPSS Statistics 19. The quantitative values between the two groups were compared by independent sample T-test. The quantitative values between multiple groups were compared by one-way ANOVA, and the two groups were compared by LSD and S–N–K methods. P < 0.05 indicates the significant difference.
3. Results
3.1. Immunofluorescence identification of fibroblasts
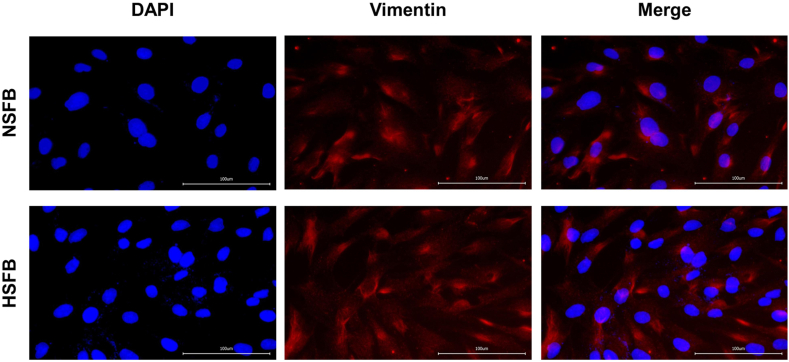
Vimentin is a marker of fibroblasts. As shown in Fig. 1, immunofluorescence staining showed that Vimentin was expressed in human HSFB and NSFB.
Fig. 1.
The hypertrophic scar fibroblasts (HSFB) and normal skin fibroblasts (NSFB) were identified by detecting the expression of Vimentin using the method of immunofluorescence ( × 400).
3.2. Expression of ClC-3 and proteins in related signal pathways was increased in scar tissues
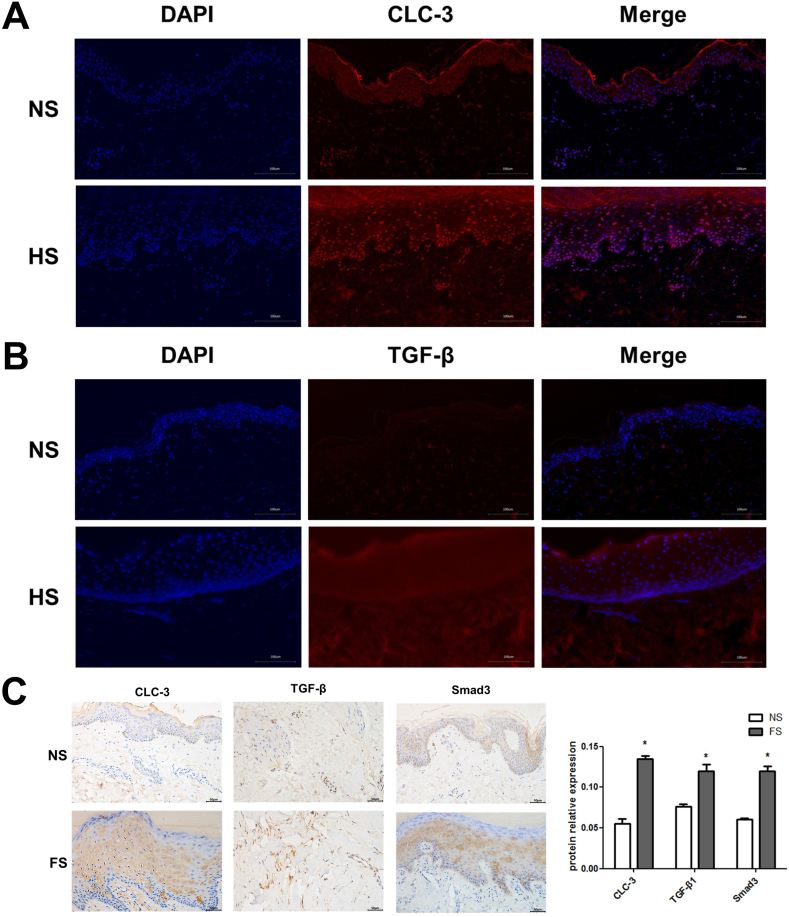
As shown in Fig. 2A and B, compared with the normal tissue, the normal structure of skin tissue disappeared, the cells were in diffused distribution, and the positive expression of ClC-3 and TGF-β was significantly increased in the scar tissue. As shown in Fig. 2C, compared with the normal tissue, the positive expression of ClC-3 (0.1351 ± 0.0077), TGF-β1 (0.1195 ± 0.0191), Smad3 (0.1196 ± 0.0145) in the scar tissue was significantly increased.
Fig. 2.
Expression of ClC-3, TGF-β and proteins in related signal pathways in scar tissues. (A) Immunofluorescence staining of ClC-3. (B) Immunofluorescence staining of TGF-β. (C) Immunohistochemical staining of ClC-3, TGF-β1, Smad3. Magnification, ×400.
3.3. Expression of ClC-3 and proteins in related signal pathways was increased in HSFB cells
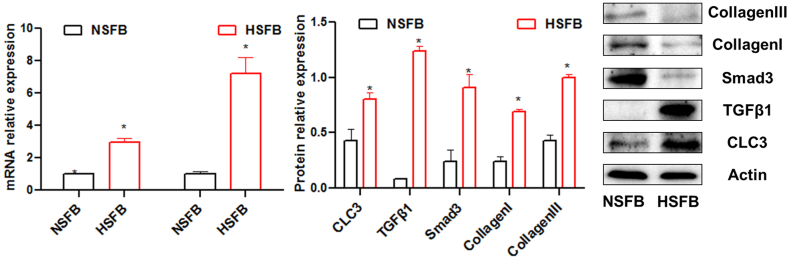
As shown in Fig. 3, qPCR results showed that compared with NSFB group, the mRNA relative expression levels of TGF-β1 (2.967 ± 0.248)and Smad3 (7.227 ± 0.991)were increased significantly in HSFB. Western blot results showed that the protein relative expression levels of ClC-3 (0.805 ± 0.062), TGF-β1 (1.237 ± 0.045), Smad3 (0.910 ± 0.117), Collagen Ⅰ(0.694 ± 0.016) and Collagen Ⅲ(0.995 ± 0.036) were increased significantly in HSFB group.
Fig. 3.
Expression of ClC-3, TGF-β and Smad3 in HSFB cells. *P < 0.05 compared with NSFB. HSFB, hypertrophic scar fibroblasts; NSFB, normal skin fibroblasts.
3.4. Effect of inhibition of CLC-3 expression on HSFB cells
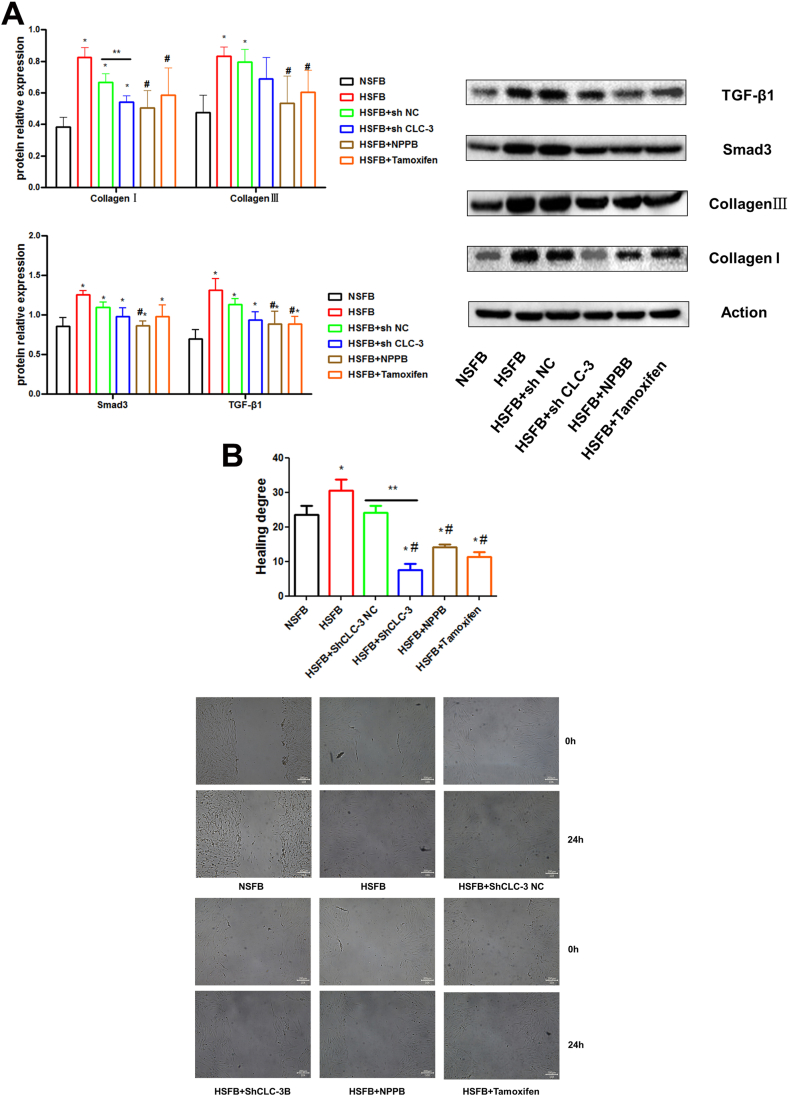
As shown in Fig. 4A, compared with the sh NC group, the expression of Collagen I proteins (0.543 ± 0.039) significantly decreased after interference with CLC-3. Compared with the HSFB group, the chloride channel blockers NPPB significantly inhibited the expression of CollagenⅠ(0.504 ± 0.111), CollagenⅢ(0.536 ± 0.170), Smad3 (0.865 ± 0.057), TGF-β1 (0.888 ± 0.157)protein, and Tamoxifen significantly inhibited the expression of CollagenⅠ(0.585 ± 0.173), CollagenⅢ(0.603 ± 0.142), TGF-β1 (0.886 ± 0.096) protein.
Fig. 4.
Effect of inhibition of CLC-3 expression on HSFB cells. (A) The expression of fibrosis-related proteins. (B) Effects of inhibition of CLC-3 expression on HSFB cells migration. *P < 0.05 compared with NSFB; #P < 0.05 compared with HSFB; **P < 0.05 compared with sh NC.
As shown in Fig. 4B, compared with the sh NC group (24.2 ± 3.67 %), the migration ability of cells significantly decreased after interference with CLC-3. Compared with the HSFB group (30.5 ± 6.51 %), the ability of cell migration in the chloride channel blocker NPPB(14.1 ± 1.61 %)and Tamoxifen (11.4 ± 2.76 %) treatment groups decreased significantly.
4. Discussion
The formation of hypertrophic scar is mainly the result of skin fibrosis caused by fibroblast migration and proliferation, and tissue fibrosis is an important reason for the formation of hypertrophic scar [6]. In general, transforming growth factor-β1 (TGF-β) is considered to be the key mediators promoting fibrosis in fibrotic diseases, and three types of TGF-β subtypes have been identified in mammals, called TGF-β1, 2 and 3 [10,11]. TGF-β1 can promote fibroblast migration and proliferation, and promote hypertrophic scar fibrosis, which is one of the important factors causing scar formation. Chlorine-3 (ClC-3) is a member of the chlorine-channel superfamily, which can participate in the regulation of various cell functions, including apoptosis, autophagy, cell cycle, etc. [12,13]. ClC-3 can promote the proliferation and migration of tumor cells [14,15]. mu et al. found that interference with the expression of CLC-3 can inhibit the division and proliferation of tumor cells through the Wnt/β-catenin signaling pathway [14]. It has been reported that ClC-3 can promote the differentiation of fibroblasts into myofibroblasts [16]. However, the regulatory role of CLC-3 in hypertrophic scar formation remains unclear.
Smad3 is a key regulator of the typical TGF-β signaling pathway, playing an important role in the transcriptional regulation mediated by TGF-β1 [17]. In many diseases, TGF-β1 activates Smad3 and leads to tissue fibrosis [10,18]. Smad3 directly combined with the promoter region of Collagen to trigger the production of collagen fibers, and inhibited the degradation of ECM (extracellular matrix) by inducing TIMP-1, while reducing the activity of MMP-1 in fibroblasts [[19], [20], [21], [22]]. In the process of renal fibrosis in rats induced by unilateral ureteral obstruction (UUO), the expression of TGF-β\ Smad3 in renal tissues was significantly increased [23]. However, Smad3-knockout mice alleviated renal fibrosis after UUO by blocking epithelial-mesenchymal cell transformation (EMT), inhibiting monocyte inflow, and collagen deposition [24]. Collagen I/Ⅲ is closely related to tissue fibrosis. Collagen II is an important component of ECM, and increased expression of Collagen II was found in liver and heart fibrosis [[25], [26], [27]]. Therefore, Collagen I/Ⅲ plays an important role in the fibrosis process of hypertrophic scars. In order to further investigate the pathways involved in hypertrophic scar formation mediated by CLC-3 chloride ion channels, we analyzed the expression of CLC-3, TGF-β1, and Smad3 in hypertrophic scar tissue and hypertrophic scar fibroblast HSFB. Our results showed that: The expression of CLC-3, TGF-β1 and Smad3 increased in hypertrophic scar tissue compared with normal tissue. Compared with NSFB, the expressions of CLC-3, TGF-β1, Smad3, CollagenⅠ\Ⅲ were increased in HSFB cells.
At the same time, knockdown of the expression of CLC-3 inhibited the cell migration ability of human hypertrophic scar fibroblasts HSFB, and the expression of CollagenⅠ in HSFB cells was inhibited. The expressions of TGF-β1, Smad3, CollagenⅠ\Ⅲ were inhibited by CLC-3 chloride channel blockers NPPB and Tamoxifen, and the migration ability of HSFB cells was decreased. Therefore, inhibition of CLC-3 can reduce HSFB cell migration ability by inhibiting TGF-β-smad3 signaling pathway to reduce hypertrophic scar formation.
Ethic statement
Research experiments conducted in this article with animals were approved by the Ethical Committee and responsible authorities of our research organization following all guidelines, regulations, legal, and ethical standards as required for animals.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81760344).
Data availability statement
Data will be available by request to the corresponding author.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Qian Liang: Writing – original draft, Formal analysis, Data curation. Fuqiang Pan: Methodology, Investigation, Data curation. Houhuang Qiu: Software, Methodology. Xiang Zhou: Visualization, Software, Methodology. Jieyun Cai: Methodology, Formal analysis. Ruijin Luo: Resources, Investigation. Zenghui Xiong: Methodology, Investigation, Formal analysis. Huawei Yang: Validation, Supervision, Conceptualization. Liming Zhang: Writing – review & editing, Visualization, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Huawei Yang, Email: Lordyhw@163.com.
Liming Zhang, Email: zlm3277902@163.com.
References
- 1.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: a 2020 update of the algorithms published 10 Years ago. Plast. Reconstr. Surg. 2022;149(1):79e–94e. doi: 10.1097/PRS.0000000000008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao Y., Guo Z., Yang Y., Liu L., Huang J., Chen Y., Li L., Sun B. Neutrophil extracellular traps contribute to myofibroblast differentiation and scar hyperplasia through the Toll-like receptor 9/nuclear factor Kappa-B/interleukin-6 pathway. Burns & trauma. 2022;10 doi: 10.1093/burnst/tkac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-beta: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016;12(6):325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 4.Lodyga M., Hinz B. TGF-beta1 - a truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020;101:123–139. doi: 10.1016/j.semcdb.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y.Y., Dou J.Y., Huang X.R., Liu X.S., Lan H.Y. Transforming growth factor-beta and long non-coding RNA in renal inflammation and fibrosis. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.684236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T., Wang X.F., Wang Z.C., Lou D., Fang Q.Q., Hu Y.Y., Zhao W.Y., Zhang L.Y., Wu L.H., Tan W.Q. Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- 7.Fang X., Hu X., Zheng Z., Tao K., Wang H., Guan H., Shi J., Ji P., Cai W., Bai X., Zhu X., Han J., Liu J., Hu D. Smad interacting protein 1 influences transforming growth factor-beta(1)/Smad signaling in extracellular matrix protein production and hypertrophic scar formation. J. Mol. Histol. 2019;50(6):503–514. doi: 10.1007/s10735-019-09844-w. [DOI] [PubMed] [Google Scholar]
- 8.Okada Y., Okada T., Sato-Numata K., Islam M.R., Ando-Akatsuka Y., Numata T., Kubo M., Shimizu T., Kurbannazarova R.S., Marunaka Y., Sabirov R.Z. Cell volume-activated and volume-correlated anion channels in mammalian cells: their biophysical, molecular, and pharmacological properties. Pharmacol. Rev. 2019;71(1):49–88. doi: 10.1124/pr.118.015917. [DOI] [PubMed] [Google Scholar]
- 9.Peng J., Chen W., Chen J., Yuan Y., Zhang J., He Y. Overexpression of chloride channel-3 predicts unfavorable prognosis and promotes cellular invasion in gastric cancer. Cancer Manag. Res. 2018;10:1163–1175. doi: 10.2147/CMAR.S159790. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Yu X.Y., Sun Q., Zhang Y.M., Zou L., Zhao Y.Y. TGF-beta/Smad signaling pathway in tubulointerstitial fibrosis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.860588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng X.M., Tang P.M., Li J., Lan H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentsch T.J., Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol. Rev. 2018;98(3):1493–1590. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 13.Hong S., Bi M., Wang L., Kang Z., Ling L., Zhao C. CLC-3 channels in cancer. Oncol. Rep. 2015;33(2):507–514. doi: 10.3892/or.2014.3615. (review) [DOI] [PubMed] [Google Scholar]
- 14.Mu H., Mu L., Gao J. Suppression of CLC-3 reduces the proliferation, invasion and migration of colorectal cancer through Wnt/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2020;533(4):1240–1246. doi: 10.1016/j.bbrc.2020.09.125. [DOI] [PubMed] [Google Scholar]
- 15.Li M., Wu D.B., Wang J., Chen L. CLC-3 Cl- channel-mediated invasion and migration of human ovarian cancer cells. Eur. J. Gynaecol. Oncol. 2016;37(5):689–695. [PubMed] [Google Scholar]
- 16.Sun L., Dong Y., Zhao J., Yin Y., Zheng Y. The CLC-2 chloride channel modulates ECM synthesis, differentiation, and migration of human conjunctival fibroblasts via the PI3K/akt signaling pathway. Int. J. Mol. Sci. 2016;17(6) doi: 10.3390/ijms17060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang P.M., Zhou S., Meng X.M., Wang Q.M., Li C.J., Lian G.Y., Huang X.R., Tang Y.J., Guan X.Y., Yan B.P., To K.F., Lan H.Y. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat. Commun. 2017;8 doi: 10.1038/ncomms14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Y.Y., Liu X.S., Huang X.R., Yu X.Q., Lan H.Y. Diverse role of TGF-beta in kidney disease. Front. Cell Dev. Biol. 2020;8:123. doi: 10.3389/fcell.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu F., Liu C., Zhou D., Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016;64(3):157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walton K.L., Johnson K.E., Harrison C.A. Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Liu C., Meng X.M., Huang C., Xu F., Li J. Smad2 protects against TGF-beta1/Smad3-mediated collagen synthesis in human hepatic stellate cells during hepatic fibrosis. Mol. Cell. Biochem. 2015;400(1–2):17–28. doi: 10.1007/s11010-014-2258-1. [DOI] [PubMed] [Google Scholar]
- 22.Hu H.H., Chen D.Q., Wang Y.N., Feng Y.L., Cao G., Vaziri N.D., Zhao Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Tian F., Zhang Z.Y., Sun J., Han Y.C. Expression of miR-207 in renal tissue of renal fibrosis rats and its correlation analysis with protein expression of TGF-beta1 and Smad3. Eur. Rev. Med. Pharmacol. Sci. 2021;25(2):787–794. doi: 10.26355/eurrev_202101_24641. [DOI] [PubMed] [Google Scholar]
- 24.Xu M., Li S., Wang J., Huang S., Zhang A., Zhang Y., Gu W., Yu X., Jia Z. Cilomilast ameliorates renal tubulointerstitial fibrosis by inhibiting the TGF-beta1-smad2/3 signaling pathway. Front. Med. 2020;7 doi: 10.3389/fmed.2020.626140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei L., Hu H., Lei Y., Feng J. Leukocytic toll-like receptor 2 knockout protects against diabetes-induced cardiac dysfunction. Biochem. Biophys. Res. Commun. 2018;506(3):668–673. doi: 10.1016/j.bbrc.2018.10.082. [DOI] [PubMed] [Google Scholar]
- 26.Clift C.L., Su Y.R., Bichell D., Jensen Smith H.C., Bethard J.R., Norris-Caneda K., Comte-Walters S., Ball L.E., Hollingsworth M.A., Mehta A.S., Drake R.R., Angel P.M. Collagen fiber regulation in human pediatric aortic valve development and disease. Sci. Rep. 2021;11(1):9751. doi: 10.1038/s41598-021-89164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karsdal M.A., Daniels S.J., Holm Nielsen S., Bager C., Rasmussen D.G.K., Loomba R., Surabattula R., Villesen I.F., Luo Y., Shevell D., Gudmann N.S., Nielsen M.J., George J., Christian R., Leeming D.J., Schuppan D. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. : official journal of the International Association for the Study of the Liver. 2020;40(4):736–750. doi: 10.1111/liv.14390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available by request to the corresponding author.