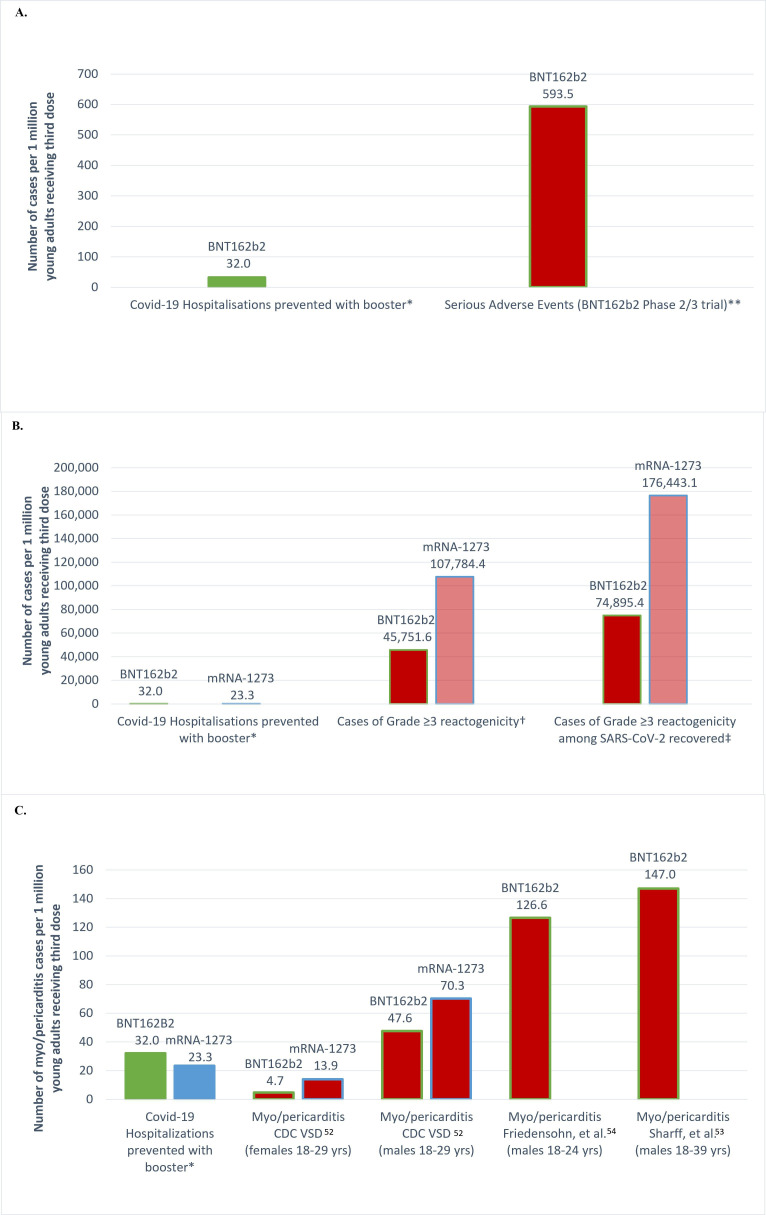
Figure 1.
(A, B, C) Expected hospitalisations prevented over six months and serious adverse events (SAEs), cases of grade ≥3 reactogenicity, and vaccine-associated myo/pericarditis among 18–29-year-olds per million BNT162b2 and mRNA-1273 booster vaccinations. *CDC-estimated number needed to vaccinate (NNV) with a booster to prevent 1 hospitalisation over 6 months in 18–29-year-olds18 was adjusted for reduced Omicron severity (aOR=0.28)47 as follows: BNT162b2 (8738/0.28=31 207) and mRNA-1273 (11 994/0.28=42 836). Per million third doses, hospitalisations prevented for BNT162b2 were computed as follows: 1/(8738/0.28)×106=1/31 207×106=32.0 and 1/(11 994/0.28)×106=1/42 836×106=23.3 for mRNA-1273 **SAEs: Three serious adverse events among BNT162b2 booster recipients were deemed by blinded investigators to be related to vaccination (3/5055). These included: moderate persistent tachycardia, moderate transient elevated hepatic enzymes, and mild elevated hepatic enzymes.18 50 †Reactogenicity rates are BNT162b2 (14/306) and 45 751.6 per million third doses; mRNA-1273 (18/167) and 107 784.4 per million third doses.50 ‡Estimated reactogenicity rates were computed assuming 63.7% seroprevalence13 and at least 2x reactogenicity among those with prior SARS-CoV-2 infection.56 57