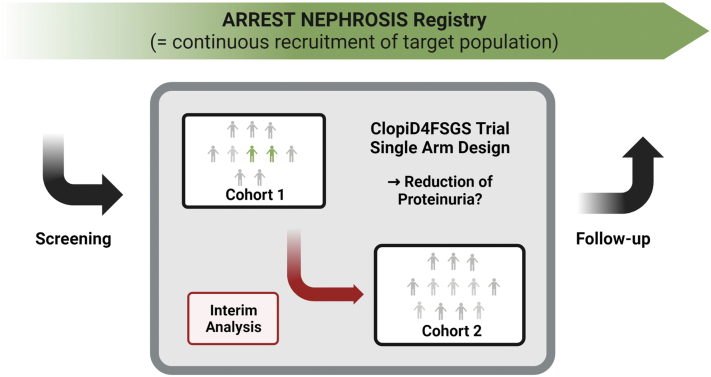
Figure 1.
ClopiD4FSGS trial overview. Patients enrolled in the ARREST NEPHROSIS registry are screened for eligibility. A total of up to 22 patients will be recruited. After the recruitment of 10 patients (= cohort 1), an interim analysis for early futility will be performed after visit 2 (= 84 days after baseline visit). The futility assessment will be based on a secondary parameter only. If no more than 1 patient reaches the focal segmental glomerulosclerosis partial remission end point, the study will be stopped for early futility. Otherwise, recruitment of the remaining patients will be completed, and the primary end point be tested in the final analysis of 22 patients. The primary outcome is the percentage change of the urinary protein-to-creatinine ratio from baseline to week 24. Focal segmental glomerulosclerosis partial remission end point is defined as a urinary protein-to-creatinine ratio <1.5 and a >40% reduction in urinary protein-to-creatinine ratio compared to baseline, which will be reported for each visit. Registry data will be continuously obtained during and following the ClopiD4FSGS trial.