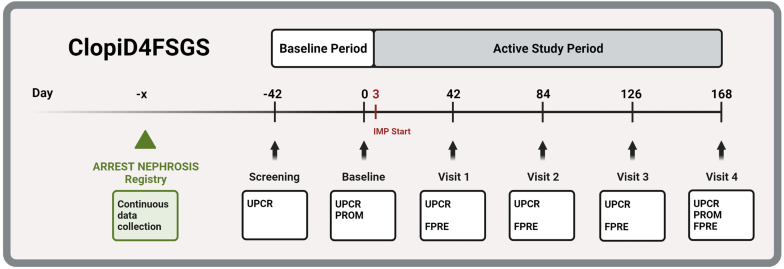
Figure 2.
ClopiD4FSGS study design. The baseline visit will be conducted 7 weeks after the screening assessment (= baseline period), followed by 24-week treatment period with 75 mg clopidogrel daily (= active study period). UPCR as assessed by 24-hour-urine collections will be assessed at baseline and at each study visit. The primary end point is defined as the percent proteinuria change from baseline to visit 4 (week 24). Individual patient trajectories will be reported. The secondary end point is the proportion of patients who achieve the FPRE after visit 4 (week 24). As tertiary end points, core outcomes as defined by the “Standardized Outcomes in Nephrology” (SONG) initiative will be assessed, including PROM for life participation. Patients are recruited from the ARREST-NEPHROSIS registry (green triangle, green box), registry data will be continuously obtained during and following the ClopiD4FSGS trial. IMP, investigational medicinal product; PROM, patient reported outcome measures; UPCR, urinary protein-to-creatinine ratio.
FPRE is defined as UPCR <1.5 and a >40% reduction in UPCR.