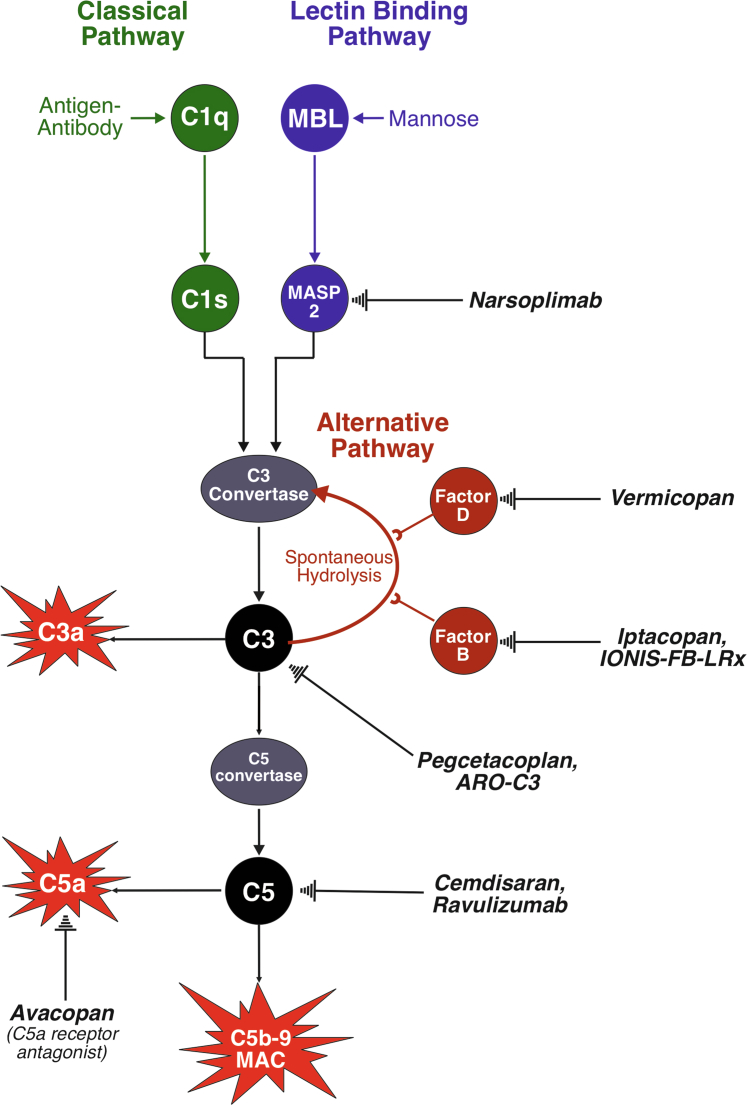
Figure 2.
The complement system. The complement system can be activated by the classical, lectin, or alternative pathways. The classical pathway (green) is activated by antigen-antibody complexes, which bind C1q allowing C1r to cleave C1s which eventually leads to the formation of a C3 convertase downstream. There is little evidence of complement system activation via the classical pathway in IgAN. The lectin pathway (blue) is activated by mannose moieties commonly found on microbial surfaces, but also on Gd-IgA1, which bind mannose binding lectin to activate MASP1 and MASP2. MASP2 activation mirrors that of C1s, eventually leading to the formation of a C3 convertase. MASP2 is inhibited by narsoplimab. The alternative pathway (red) is constantly activated by the hydrolysis of C3; the pathway is promoted by factor B and factor D. Factor B is inhibited by iptacopan and IONIS-FB-LRx, whereas factor D is inhibited by vermicopan. The common pathway (black) is activated by any C3 convertase, cleaving C3 (inhibited by pegcetacoplan and ARO-C3) to C3a, an inflammatory mediator, and C3b. C3b is further acted upon by the C3 convertases to produce a C5 convertase, which cleaves C5 (can be inhibited by cemdisaran and ravuluzimab) to produce C5a, an inflammatory mediator (the C5a receptor can be blocked by avacopan), and C5b. This eventually leads to the formation of the membrane attack complex, which is capable of cell lysis. Gd-IgA1, galactose deficient-IgA1; IgAN, IgA nephropathy; MASP1, mannose-binding lectin serine protease 1; MASP2, mannose-binding lectin serine protease 2.