Figure 1: Participant recruitment and disposition.
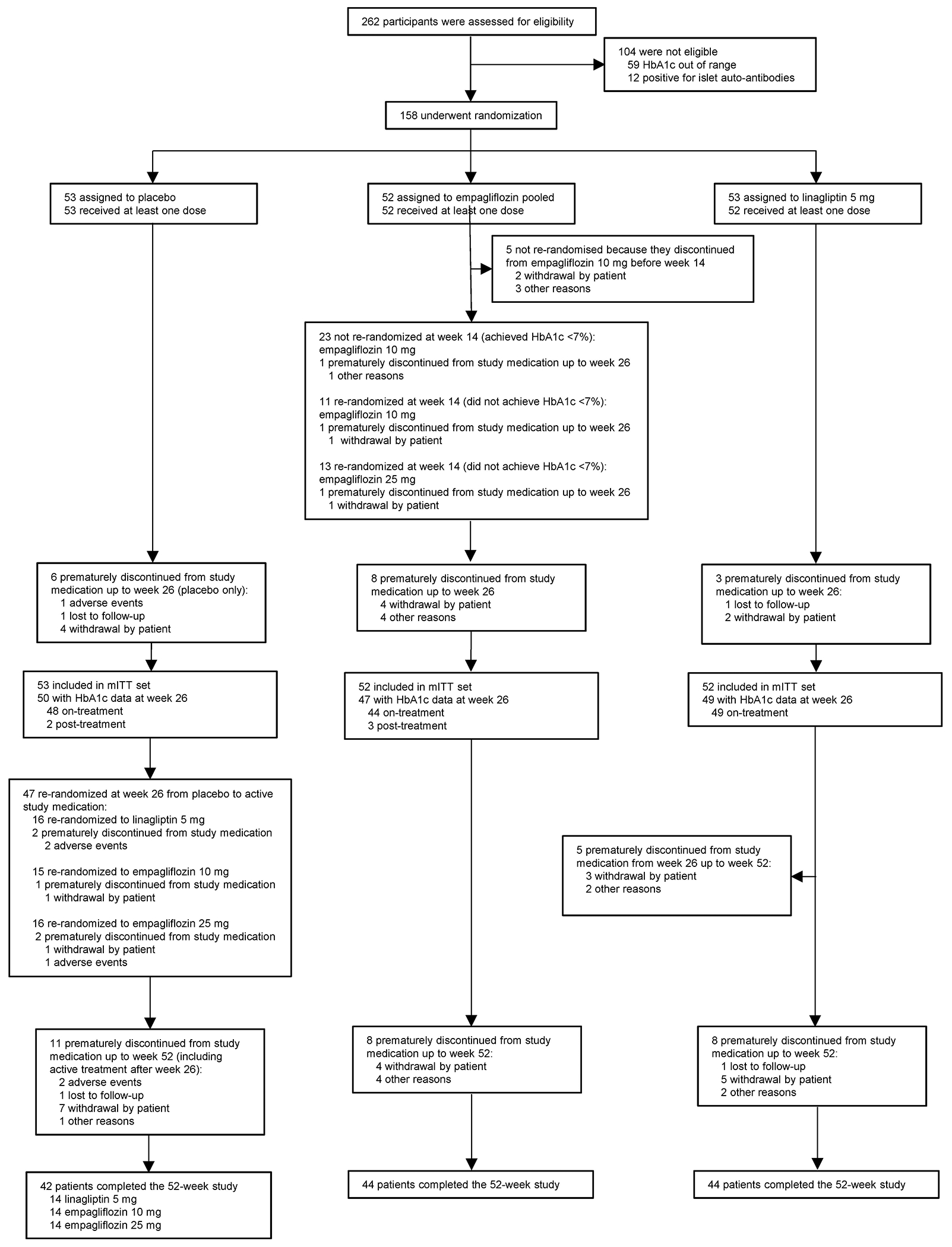
TG1 population.
Among those discontinuing treatment before week 26, on-treatment HbA1c was available for 1 participant.
Reasons for discontinuation of empagliflozin treatment described as ‘other reasons’ included:
1. Exclusionary lab was received more than 2 weeks after randomisation, participant was discontinued per sponsor.
2. Principal investigator decision to discontinue due to non-compliance.
3. Participant has decided to stop taking study medication and, following the study protocol, participant was willing to attend visit 8 only.
4. Despite many attempts to contact participant, person lost to follow-up.
HbA1c, glycated haemoglobin; mITT, modified intention-to-treat; TG, treatment groupings (see Treatment group definitions in this Supplementary Appendix for detailed explanation).
