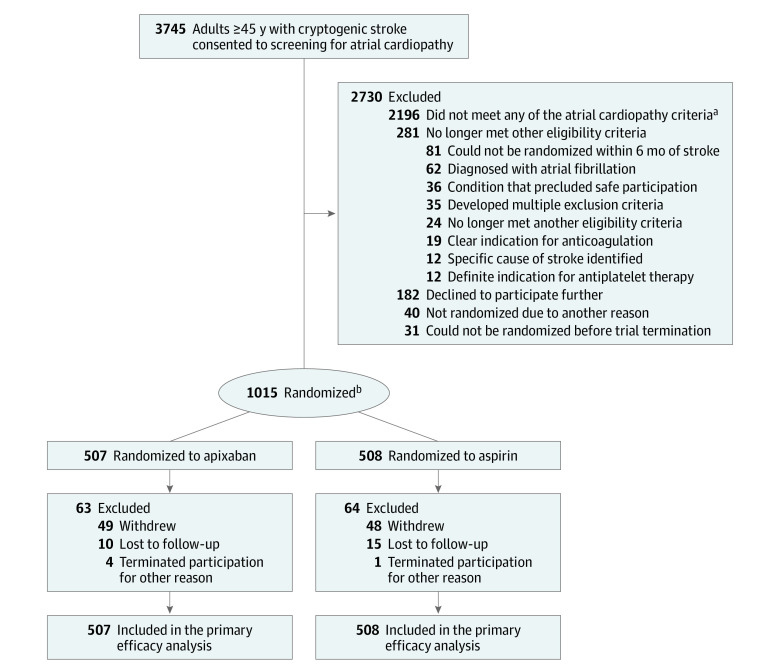
Figure 1. Enrollment, Treatment Allocation, and Analysis in the ARCADIA Trial.
ARCADIA indicates Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke.
aAtrial cardiopathy was defined as at least 1 of the following biomarkers: P-wave terminal force in electrocardiogram lead V1 >5000 μV × ms, serum N-terminal pro-B-type natriuretic peptide level >250 pg/mL, or left atrial diameter index ≥3 cm/m2 on echocardiogram.
bEligible participants were randomly assigned in a 1:1 ratio to apixaban or aspirin using a central randomization system and a method that controlled the treatment imbalance within each StrokeNet Regional Coordinating Center. One patient was incorrectly randomized despite not meeting the atrial cardiopathy biomarker criteria.