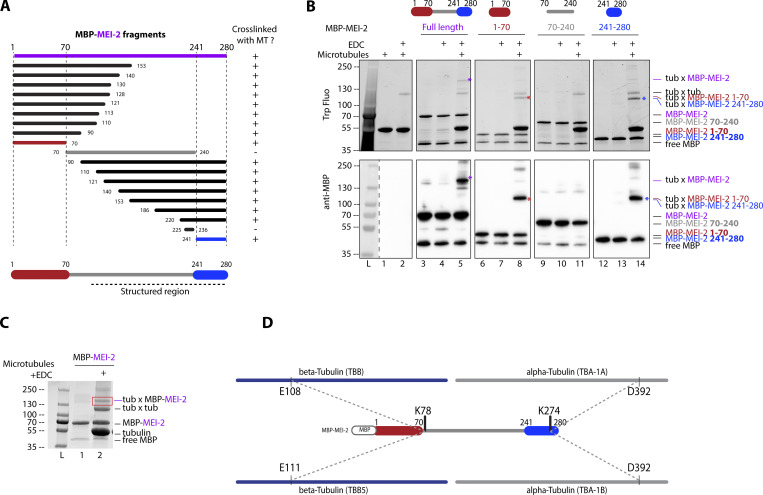
Figure 2.
Katanin binds to microtubules via two domains on MEI-2 subunit. (A) Schematic representation of all MEI-2 fragments tagged with MBP tested in crosslink with microtubules in vitro. Black fragments still crosslink with microtubules, whereas the gray fragment does not. MEI-2 1–70 (red) and MEI-2 241–280 (blue) were identified as two minimal MEI-2 fragments able to crosslink with microtubules. The dashed line corresponds to the putative structured region of MEI-2 based on the Mm Katnb1 fragment structure (Jiang et al., 2018). (B) Crosslink assay between MBP–MEI-2 full-length (purple) or fragments and microtubules. Colorful stars indicate crosslinked complexes MBP–MEI-2*tubulin or MBP-MEI-2 fragment*tubulin. SDS PAGE was analyzed using tryptophan fluorescence (Stain Free; Bio-Rad; upper panel) and by Western blot using anti-MBP antibody (lower panel). The absence of crosslink between MBP tag alone and microtubules is controlled in Fig. S2. Experiments were performed at least in triplicate and the maximal variation observed was <10%. (C) Red square corresponds to crosslink complex MBP–MEI-2*tubulin extracted from SDS PAGE to be analyzed by mass spectrometry. (D) Schematic representation of MEI-2, porcine alpha- and beta-tubulins crosslinked residues identified by mass-spectrometry (see Table S1). Two MEI-2 residues were identified as specifically crosslinked with alpha- or beta-tubulin. Source data are available for this figure: SourceData F2.