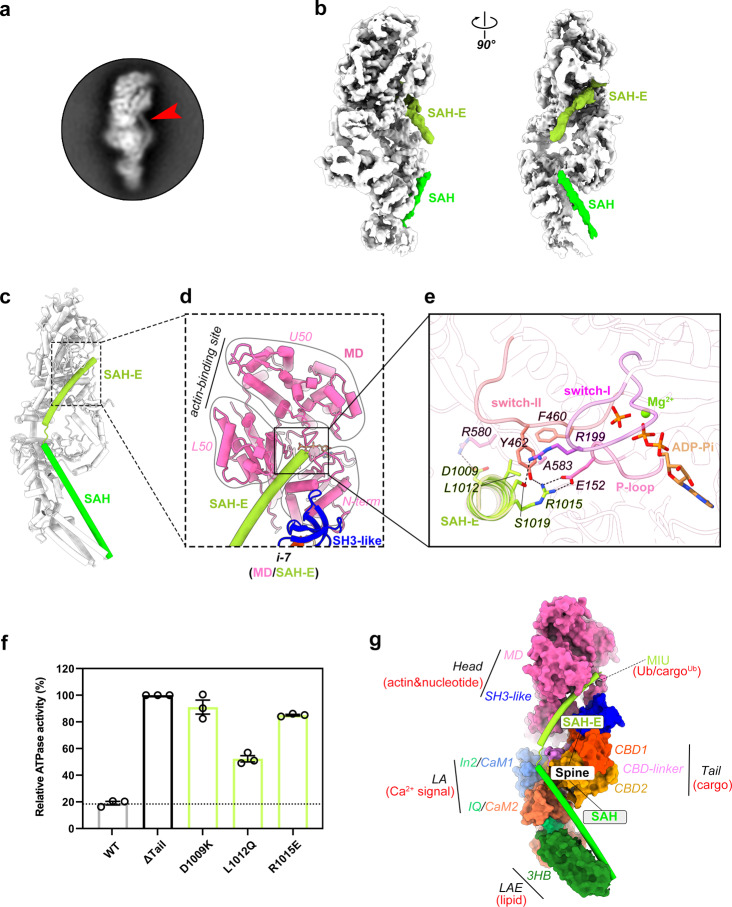
Fig. 3. The “spine”-like structure formed by the SAH and SAH-E in blocking the active site and integrating myosin VI domains.
a Representation of a 2D class of myosin VI particles showing a stick-like density extending from the end of SAH, indicated by a red arrowhead. b, c Cryo-EM map with contour level of 0.1. (b) and atomic model (c) of autoinhibited myosin VI with the SAH and SAH-E highlighted. The SAH-E helix is inserted into a cavity in the MD. d Interface-7 between the SAH-E and MD in autoinhibited myosin VI. The cavity is formed by the SH3-like domain and the N-term and L50 subdomains in the MD. The actin-binding site between U50 and L50 is indicated. e Detailed depiction of the molecular interaction at interface-7. The C-terminal of SAH-E interacts directly with essential elements (P-loop, switch-I, switch-II) of the active site in the MD. f In vitro ATPase activity measurements for three myosin VI mutants with the impaired SAH-E/MD interaction. The measured activity of the ΔTail mutant is set as 100 % activation of myosin VI and other samples are normalized to it for comparison. g Representation of the SAH and SAH-E with the surrounding regions in the autoinhibited state of myosin VI. These two consecutive single α-helices link the head, LA, LAE, and tail together. Various factors, such as ubiquitin (Ub), ubiquitinated cargo (cargoUb), Ca2+, and lipids that interfere with this linkage potentially release the autoinhibition of myosin VI.